D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation
- PMID: 30792823
- PMCID: PMC6376546
- DOI: 10.1177/1758835918821021
D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation
Abstract
Background: There are close links between chemotherapy-induced intestinal mucositis and microbiota dysbiosis. Previous studies indicated that D-methionine was an excellent candidate for a chemopreventive agent. Here, we investigated the effects of D-methionine on cisplatin-induced mucositis.
Materials and methods: Male Wistar rats (176-200 g, 6 weeks old) were given cisplatin (5 mg/kg) and treated with D-methionine (300 mg/kg). Histopathological, digestive enzymes activity, oxidative/antioxidant status, proinflammatory/anti-inflammatory cytokines in intestinal tissues were measured. Next-generation sequencing technologies were also performed to investigate the gut microbial ecology.
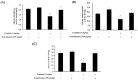
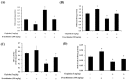
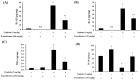
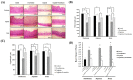
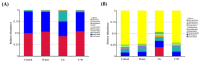
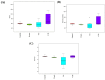
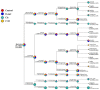
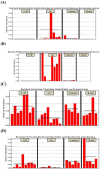
Results: D-methionine administration increased villus length and crypt depth and improved digestive enzyme (leucine aminopeptidase, sucrose and alkaline phosphatase) activities in the brush-border membrane of cisplatin-treated rats (p < 0.05). Furthermore, D-methionine significantly attenuated oxidative stress and inflammatory reaction and increased interleukin-10 levels in cisplatin-induced intestinal mucositis (p < 0.05). Cisplatin administration resulted in high relative abundances of Deferribacteres and Proteobacteria and a low diversity of the microbiota when compared with control groups, D-methionine only and cisplatin plus D-methionine. Cisplatin markedly increased comparative abundances of Bacteroides caccae, Escherichia coli, Mucispirillum schaedleri, Bacteroides uniformis and Desulfovibrio C21-c20, while Lactobacillus was almost completely depleted, compared with the control group. There were higher abundances of Lactobacillus, Lachnospiraceae, and Clostridium butyrium in cisplatin plus D-methionine rats than in cisplatin rats. D-methionine treatment alone significantly increased the number of Lactobacillus reuteri.
Conclusion: D-methionine protects against cisplatin-induced intestinal damage through antioxidative and anti-inflammatory effects. By enhancing growth of beneficial bacteria (Lachnospiraceae and Lactobacillus), D-methionine attenuates gut microbiome imbalance caused by cisplatin and maintains gut homeostasis.
Keywords: D-methionine; Lactobacillus; cisplatin; gastrointestinal mucositis; next-generation sequencing.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures

References
-
- Montassier E, Gastinne T, Vangay P, et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther 2015; 42: 515–528. - PubMed
-
- Wang C, Yang S, Gao L, et al. Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice. Food Funct 2018; 9: 2695–2704. - PubMed
-
- Touchefeu Y, Montassier E, Nieman K, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 2014; 40: 409–421. - PubMed
LinkOut - more resources
Full Text Sources

