Haploinsufficiency of autism causative gene Tbr1 impairs olfactory discrimination and neuronal activation of the olfactory system in mice
- PMID: 30792833
- PMCID: PMC6371489
- DOI: 10.1186/s13229-019-0257-5
Haploinsufficiency of autism causative gene Tbr1 impairs olfactory discrimination and neuronal activation of the olfactory system in mice
Abstract
Background: Autism spectrum disorders (ASD) exhibit two clusters of core symptoms, i.e., social and communication impairment, and repetitive behaviors and sensory abnormalities. Our previous study demonstrated that TBR1, a causative gene of ASD, controls axonal projection and neuronal activation of amygdala and regulates social interaction and vocal communication in a mouse model. Behavioral defects caused by Tbr1 haploinsufficiency can be ameliorated by increasing neural activity via D-cycloserine treatment, an N-methyl-D-aspartate receptor (NMDAR) coagonist. In this report, we investigate the role of TBR1 in regulating olfaction and test whether D-cycloserine can also improve olfactory defects in Tbr1 mutant mice.
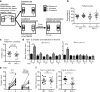
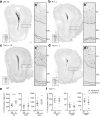
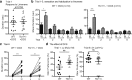
Methods: We used Tbr1+/- mice as a model to investigate the function of TBR1 in olfactory sensation and discrimination of non-social odors. We employed a behavioral assay to characterize the olfactory defects of Tbr1+/- mice. Magnetic resonance imaging (MRI) and histological analysis were applied to characterize anatomical features. Immunostaining was performed to further analyze differences in expression of TBR1 subfamily members (namely TBR1, TBR2, and TBX21), interneuron populations, and dendritic abnormalities in olfactory bulbs. Finally, C-FOS staining was used to monitor neuronal activation of the olfactory system upon odor stimulation.
Results: Tbr1+/- mice exhibited smaller olfactory bulbs and anterior commissures, reduced interneuron populations, and an abnormal dendritic morphology of mitral cells in the olfactory bulbs. Tbr1 haploinsufficiency specifically impaired olfactory discrimination but not olfactory sensation. Neuronal activation upon odorant stimulation was reduced in the glomerular layer of Tbr1+/- olfactory bulbs. Furthermore, although the sizes of piriform and perirhinal cortices were not affected by Tbr1 deficiency, neuronal activation was reduced in these two cortical regions in response to odorant stimulation. These results suggest an impairment of neuronal activation in olfactory bulbs and defective connectivity from olfactory bulbs to the upper olfactory system in Tbr1+/- mice. Systemic administration of D-cycloserine, an NMDAR co-agonist, ameliorated olfactory discrimination in Tbr1+/- mice, suggesting that increased neuronal activity has a beneficial effect on Tbr1 deficiency.
Conclusions: Tbr1 regulates neural circuits and activity in the olfactory system to control olfaction. Tbr1+/- mice can serve as a suitable model for revealing how an autism causative gene controls neuronal circuits, neural activity, and autism-related behaviors.
Keywords: Autism spectrum disorders; C-FOS; D-cycloserine; Neuronal activation; Olfactory bulb; Olfactory discrimination; T-brain-1.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. - PubMed
-
- Mandy WP, Charman T, Skuse DH. Testing the construct validity of proposed criteria for DSM-5 autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):41–50. - PubMed
-
- Sztainberg Y, Zoghbi HY. Lessons learned from studying syndromic autism spectrum disorders. Nat Neurosci. 2016;19(11):1408–1417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

