Fight them or feed them: how the intestinal mucus layer manages the gut microbiota
- PMID: 30792861
- PMCID: PMC6375348
- DOI: 10.1093/gastro/goy052
Fight them or feed them: how the intestinal mucus layer manages the gut microbiota
Abstract
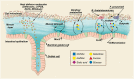
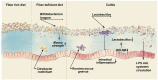
The intestinal tract is inhabited by a tremendous number of microorganisms, termed the gut microbiota. These microorganisms live in a mutualistic relationship with their host and assist in the degradation of complex carbohydrates. Although the gut microbiota is generally considered beneficial, the vast number of microbial cells also form a permanent threat to the host. Thus, the intestinal epithelium is covered with a dense layer of mucus to prevent translocation of the gut microbiota into underlying tissues. Intestinal mucus is an organized glycoprotein network with a host-specific glycan structure. While the mucus layer has long been considered a passive, host-designed barrier, recent studies showed that maturation and function of the mucus layer are strongly influenced by the gut microbiota. In return, the glycan repertoire of mucins can select for distinct mucosa-associated bacteria that are able to bind or degrade specific mucin glycans as a nutrient source. Because the intestinal mucus layer is at the crucial interface between host and microbes, its breakdown leads to gut bacterial encroachment that can eventually cause inflammation and infection. Accordingly, a dysfunctional mucus layer has been observed in colitis in mice and humans. Moreover, the increased consumption of a low-fiber Western-style diet in our modern society has recently been demonstrated to cause bacteria-mediated defects of the intestinal mucus layer. Here, I will review current knowledge on the interaction between gut bacteria and the intestinal mucus layer in health and disease. Understanding the molecular details of this host-microbe interaction may contribute to the development of novel treatment options for diseases involving a dysfunctional mucus layer, such as ulcerative colitis.
Keywords: Dietary fiber; gut microbiota; host–microbe interaction; inflammatory bowel disease; metabolic disease; mucin; mucosal barrier; mucus; probiotics; ulcerative colitis.
Figures
References
-
- Schroeder BO, Bäckhed F.. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 2016;22:1079–89. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

