Implementing TMB measurement in clinical practice: considerations on assay requirements
- PMID: 30792906
- PMCID: PMC6350758
- DOI: 10.1136/esmoopen-2018-000442
Implementing TMB measurement in clinical practice: considerations on assay requirements
Abstract
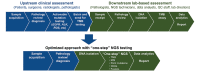
Clinical evidence demonstrates that treatment with immune checkpoint inhibitor immunotherapy agents can have considerable benefit across multiple tumours. However, there is a need for the development of predictive biomarkers that identify patients who are most likely to respond to immunotherapy. Comprehensive characterisation of tumours using genomic, transcriptomic, and proteomic approaches continues to lead the way in advancing precision medicine. Genetic correlates of response to therapy have been known for some time, but recent clinical evidence has strengthened the significance of high tumour mutational burden (TMB) as a biomarker of response and hence a rational target for immunotherapy. Concordantly, immune checkpoint inhibitors have changed clinical practice for lung cancer and melanoma, which are tumour types with some of the highest mutational burdens. TMB is an implementable approach for molecular biology and/or pathology laboratories that provides a quantitative measure of the total number of mutations in tumour tissue of patients and can be assessed by whole genome, whole exome, or large targeted gene panel sequencing of biopsied material. Currently, TMB assessment is not standardised across research and clinical studies. As a biomarker that affects treatment decisions, it is essential to unify TMB assessment approaches to allow for reliable, comparable results across studies. When implementing TMB measurement assays, it is important to consider factors that may impact the method workflow, the results of the assay, and the interpretation of the data. Such factors include biopsy sample type, sample quality and quantity, genome coverage, sequencing platform, bioinformatic pipeline, and the definitions of the final threshold that determines high TMB. This review outlines the factors for adoption of TMB measurement into clinical practice, providing an understanding of TMB assay considerations throughout the sample journey, and suggests principles to effectively implement TMB assays in a clinical setting to aid and optimise treatment decisions.
Keywords: Tumor mutational burden; assay implementation; immune checkpoint inhibitor; immunotherapy; next-generation sequencing.
Conflict of interest statement
Competing interests: ER reports personal fees and non-financial support from AstraZeneca and Bristol-Myers Squibb. JL reports grants from Agilent Technologies and Roche Tissue Diagnostics, and personal fees from AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Pfizer, and Roche Tissue Diagnostics. FL-R reports personal fees from AstraZeneca, Bristol-Myers Squibb, Life Technologies, Merck, Pfizer, and Roche. FP-L reports grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Merck, and Roche, and grants from NanoString. NN reports grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Qiagen, Roche, and Thermo Fisher Scientific, and grants and non-financial support from Merck. SM-B reports grants and personal fees from AstraZeneca and Novartis, and personal fees from Bristol-Myers Squibb and Roche. RB declares no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

