CaMello-XR enables visualization and optogenetic control of Gq/11 signals and receptor trafficking in GPCR-specific domains
- PMID: 30793039
- PMCID: PMC6376006
- DOI: 10.1038/s42003-019-0292-y
CaMello-XR enables visualization and optogenetic control of Gq/11 signals and receptor trafficking in GPCR-specific domains
Abstract
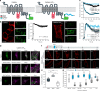
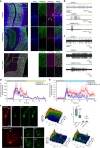
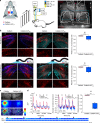
The signal specificity of G protein-coupled receptors (GPCRs) including serotonin receptors (5-HT-R) depends on the trafficking and localization of the GPCR within its subcellular signaling domain. Visualizing traffic-dependent GPCR signals in neurons is difficult, but important to understand the contribution of GPCRs to synaptic plasticity. We engineered CaMello (Ca2+-melanopsin-local-sensor) and CaMello-5HT2A for visualization of traffic-dependent Ca2+ signals in 5-HT2A-R domains. These constructs consist of the light-activated Gq/11 coupled melanopsin, mCherry and GCaMP6m for visualization of Ca2+ signals and receptor trafficking, and the 5-HT2A C-terminus for targeting into 5-HT2A-R domains. We show that the specific localization of the GPCR to its receptor domain drastically alters the dynamics and localization of the intracellular Ca2+ signals in different neuronal populations in vitro and in vivo. The CaMello method may be extended to every GPCR coupling to the Gq/11 pathway to help unravel new receptor-specific functions in respect to synaptic plasticity and GPCR localization.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Alterations in 5-HT2A receptor signaling in male and female transgenic rats over-expressing either Gq or RGS-insensitive Gq protein.Neuropharmacology. 2006 Sep;51(3):524-35. doi: 10.1016/j.neuropharm.2006.04.012. Neuropharmacology. 2006. PMID: 16769091
-
Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6479-84. doi: 10.1073/pnas.1321576111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733892 Free PMC article.
-
Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia.Sci Signal. 2016 Jan 12;9(410):ra5. doi: 10.1126/scisignal.aab0467. Sci Signal. 2016. PMID: 26758213 Free PMC article.
-
The manipulation of neural and cellular activities by ectopic expression of melanopsin.Neurosci Res. 2013 Jan;75(1):3-5. doi: 10.1016/j.neures.2012.07.010. Epub 2012 Sep 12. Neurosci Res. 2013. PMID: 22982474 Review.
-
GqPCR-mediated signalling in the spotlight: from visualization towards dissection and quantification.Curr Pharm Biotechnol. 2014;15(10):893-915. doi: 10.2174/1389201015666140922101637. Curr Pharm Biotechnol. 2014. PMID: 25248560 Review.
Cited by
-
Selective optogenetic control of Gq signaling using human Neuropsin.Nat Commun. 2022 Apr 1;13(1):1765. doi: 10.1038/s41467-022-29265-w. Nat Commun. 2022. PMID: 35365606 Free PMC article.
-
Optical Approaches for Investigating Neuromodulation and G Protein-Coupled Receptor Signaling.Pharmacol Rev. 2023 Nov;75(6):1119-1139. doi: 10.1124/pharmrev.122.000584. Epub 2023 Jul 10. Pharmacol Rev. 2023. PMID: 37429736 Free PMC article. Review.
-
Optogenetic activation of mGluR1 signaling in the cerebellum induces synaptic plasticity.iScience. 2022 Dec 17;26(1):105828. doi: 10.1016/j.isci.2022.105828. eCollection 2023 Jan 20. iScience. 2022. PMID: 36632066 Free PMC article.
-
Lamprey Parapinopsin ("UVLamP"): a Bistable UV-Sensitive Optogenetic Switch for Ultrafast Control of GPCR Pathways.Chembiochem. 2020 Mar 2;21(5):612-617. doi: 10.1002/cbic.201900485. Epub 2019 Oct 30. Chembiochem. 2020. PMID: 31468691 Free PMC article.
-
Exploring the Causal Relationship Between Migraine and Insomnia Through Bidirectional Two-Sample Mendelian Randomization: A Bidirectional Causal Relationship.J Pain Res. 2024 Jul 16;17:2407-2415. doi: 10.2147/JPR.S460566. eCollection 2024. J Pain Res. 2024. PMID: 39050680 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

