Vitamin D in malaria: more hypotheses than clues
- PMID: 30793054
- PMCID: PMC6370580
- DOI: 10.1016/j.heliyon.2019.e01183
Vitamin D in malaria: more hypotheses than clues
Abstract
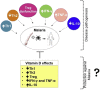
Vitamin D is a secosteroid hormone regulating calcium and phosphate metabolism, immune response and brain development. Low blood 25(OH)D levels have been reported in patients affected by infectious diseases caused by parasites, including malaria. Despite the high effectiveness of antimalarials, malaria is burdened with high morbidity and mortality, and the search for additional therapies is rapidly growing. Furthermore, available preventive measures have proved to be barely effective so far. Finding new prevention and therapy tools is a matter of urgency. Studies on animal models and humans have hypothesized some mechanisms by which the hormone can influence malaria pathogenesis, and the role of Vitamin D supplementation in preventing and treating this disease has been suggested. Few studies on the association between Vitamin D and malaria are available and disagreeing results have been reported. Studies in humans reporting an association between low 25(OH)D circulating levels and Malaria have a small sample size and observational study-set. Randomized controlled trials are needed in order to understand if Vitamin D administration might play a role in preventing and treating malaria.
Keywords: Biochemistry; Immunology; Infectious disease.
Figures
Similar articles
-
1alpha(OH)D3 One-alpha-hydroxy-cholecalciferol--an active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis.Dan Med Bull. 2008 Nov;55(4):186-210. Dan Med Bull. 2008. PMID: 19232159 Review.
-
Vitamin D and Calcium: A Systematic Review of Health Outcomes (Update).Evid Rep Technol Assess (Full Rep). 2014 Sep;(217):1-929. doi: 10.23970/AHRQEPCERTA217. Evid Rep Technol Assess (Full Rep). 2014. PMID: 30313003
-
Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth.Pediatrics. 2002 Jan;109(1):E6. doi: 10.1542/peds.109.1.e6. Pediatrics. 2002. PMID: 11773574 Clinical Trial.
-
Vitamin D supplementation for the treatment of COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 24;5(5):CD015043. doi: 10.1002/14651858.CD015043. Cochrane Database Syst Rev. 2021. PMID: 34029377 Free PMC article.
-
Vitamin D status is associated with mortality, morbidity, and growth failure among a prospective cohort of HIV-infected and HIV-exposed Tanzanian infants.J Nutr. 2015 Jan;145(1):121-7. doi: 10.3945/jn.114.201566. Epub 2014 Nov 12. J Nutr. 2015. PMID: 25527666 Free PMC article. Clinical Trial.
Cited by
-
Characterization of Vitamin D Status in Older Persons with Cognitive Impairment.Nutrients. 2022 Mar 8;14(6):1142. doi: 10.3390/nu14061142. Nutrients. 2022. PMID: 35334800 Free PMC article.
-
Vitamin D and Genetic Susceptibility to Multiple Sclerosis.Biochem Genet. 2021 Feb;59(1):1-30. doi: 10.1007/s10528-020-10010-1. Epub 2020 Nov 7. Biochem Genet. 2021. PMID: 33159645 Review.
-
The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19.J Transl Med. 2020 Aug 26;18(1):322. doi: 10.1186/s12967-020-02488-5. J Transl Med. 2020. PMID: 32847594 Free PMC article. Review.
-
Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy.Int J Mol Sci. 2022 May 25;23(11):5949. doi: 10.3390/ijms23115949. Int J Mol Sci. 2022. PMID: 35682626 Free PMC article. Review.
-
Non-Skeletal Activities of Vitamin D: From Physiology to Brain Pathology.Medicina (Kaunas). 2019 Jul 5;55(7):341. doi: 10.3390/medicina55070341. Medicina (Kaunas). 2019. PMID: 31284484 Free PMC article. Review.
References
-
- Wang T.T., Tavera-Mendoza L.E., Laparriere D. Large-scale in silico and microarray-based identification of direct 1, 25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005;19:2685–2695. - PubMed
-
- Sharif K., Sharif Y., Watad A. Vitamin D, autoimmunity and recurrent pregnancy loss: more than an association. Am. J. Reprod. Immunol. 2018;80(3) - PubMed
-
- Ali A., Cui X., Eyles D. Developmental vitamin D deficiency and autism: putative pathogenic mechanisms. J. Steroid Biochem. Mol. Biol. 2018;175:108–118. - PubMed
-
- Yamamoto K., Iwagami M., Seki T. Dual antiplasmodial activity of vitamin D3 and its analog, 22-oxacalcitriol, by direct and indirect mechanisms. Parasitrol. Int. 2017;66(2):89–99. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

