Individual- versus group-optimality in the production of secreted bacterial compounds
- PMID: 30793292
- PMCID: PMC6467250
- DOI: 10.1111/evo.13701
Individual- versus group-optimality in the production of secreted bacterial compounds
Abstract
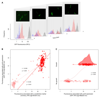
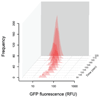
How unicellular organisms optimize the production of compounds is a fundamental biological question. While it is typically thought that production is optimized at the individual-cell level, secreted compounds could also allow for optimization at the group level, leading to a division of labor where a subset of cells produces and shares the compound with everyone. Using mathematical modeling, we show that the evolution of such division of labor depends on the cost function of compound production. Specifically, for any trait with saturating benefits, linear costs promote the evolution of uniform production levels across cells. Conversely, production costs that diminish with higher output levels favor the evolution of specialization-especially when compound shareability is high. When experimentally testing these predictions with pyoverdine, a secreted iron-scavenging compound produced by Pseudomonas aeruginosa, we found linear costs and, consistent with our model, detected uniform pyoverdine production levels across cells. We conclude that for shared compounds with saturating benefits, the evolution of division of labor is facilitated by a diminishing cost function. More generally, we note that shifts in the level of selection from individuals to groups do not solely require cooperation, but critically depend on mechanistic factors, including the distribution of compound synthesis costs.
Keywords: Bacteria; division of labor; economy of scales; group level selection; optimal production; siderophores.
© 2019 The Author(s). Evolution © 2019 The Society for the Study of Evolution.
Figures



References
-
- Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nature Publishing Group. 2015;13:497–508. - PubMed
-
- Ackermann M, Stecher B, Freed NE, Songhet P, Hardt W-D, Doebeli M. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. - PubMed
-
- Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8:450–461. - PubMed
-
- Asfahl KL, Schuster M. Social interactions in bacterial cell–cell signaling. FEMS Microbiol Rev. 2017;41:92–107. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
