Systems biology identifies preserved integrity but impaired metabolism of mitochondria due to a glycolytic defect in Alzheimer's disease neurons
- PMID: 30793475
- PMCID: PMC6516149
- DOI: 10.1111/acel.12924
Systems biology identifies preserved integrity but impaired metabolism of mitochondria due to a glycolytic defect in Alzheimer's disease neurons
Abstract
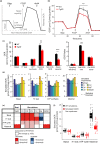
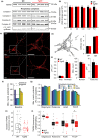
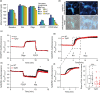
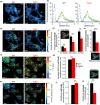
Mitochondrial dysfunction is implicated in most neurodegenerative diseases, including Alzheimer's disease (AD). We here combined experimental and computational approaches to investigate mitochondrial health and bioenergetic function in neurons from a double transgenic animal model of AD (PS2APP/B6.152H). Experiments in primary cortical neurons demonstrated that AD neurons had reduced mitochondrial respiratory capacity. Interestingly, the computational model predicted that this mitochondrial bioenergetic phenotype could not be explained by any defect in the mitochondrial respiratory chain (RC), but could be closely resembled by a simulated impairment in the mitochondrial NADH flux. Further computational analysis predicted that such an impairment would reduce levels of mitochondrial NADH, both in the resting state and following pharmacological manipulation of the RC. To validate these predictions, we utilized fluorescence lifetime imaging microscopy (FLIM) and autofluorescence imaging and confirmed that transgenic AD neurons had reduced mitochondrial NAD(P)H levels at rest, and impaired power of mitochondrial NAD(P)H production. Of note, FLIM measurements also highlighted reduced cytosolic NAD(P)H in these cells, and extracellular acidification experiments showed an impaired glycolytic flux. The impaired glycolytic flux was identified to be responsible for the observed mitochondrial hypometabolism, since bypassing glycolysis with pyruvate restored mitochondrial health. This study highlights the benefits of a systems biology approach when investigating complex, nonintuitive molecular processes such as mitochondrial bioenergetics, and indicates that primary cortical neurons from a transgenic AD model have reduced glycolytic flux, leading to reduced cytosolic and mitochondrial NAD(P)H and reduced mitochondrial respiratory capacity.
Keywords: Alzheimer's disease; glycolysis; mitochondria; neurons; systems biology.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


References
-
- Aguilar‐Arnal, L. , Ranjit, S. , Stringari, C. , Orozco‐Solis, R. , Gratton, E. , & Sassone‐Corsi, P. (2016). Spatial dynamics of SIRT1 and the subnuclear distribution of NADH species. Proceedings of the National Academy of Sciences of the United States of America, 113(45), 12715–12720. 10.1073/pnas.1609227113 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

