Dynamic alterations of plasma cell free DNA in response to chemotherapy in children with neuroblastoma
- PMID: 30793512
- PMCID: PMC6488154
- DOI: 10.1002/cam4.2045
Dynamic alterations of plasma cell free DNA in response to chemotherapy in children with neuroblastoma
Abstract
Background: To improve cure rates for neuroblastoma (NB), it is important and necessary to evaluate therapy response. Our investigation focuses on using plasma cell free DNA (cfDNA) as a biomarker to determine tumor burden and minimal residual disease (MRD) of NB patients during chemotherapy.
Methods: Total 58 NB patients were recruited from July 2016 to December 2017. Therapy regime and risk classification were based on COG standard and BCH-NB-2007 protocol. RECIST study was used to judge response to therapy at the end of fourth cycle of chemotherapy (CC4) and maintenance stage (MS) respectively. Serial quantifications of cfDNA, NSE, and LDH were examined at four stages, including newly diagnosed, second and CC4, and maintenance.
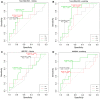
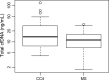
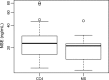
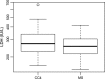
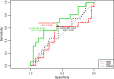
Results: During early chemotherapy, 65.5% of NB kids responded well. Consistently, cfDNA, NSE, and LDH levels were down-regulated in NB patients with partial remission (PR) compared to those with stable disease (SD). In both training and predicting sets, the levels of cfDNA were significantly comparable between PR and SD only at CC4 stage. To predict the insufficient response to early chemotherapy, the optimal AUC value of cfDNA was 0.732 and 0.747 in training and predicting sets respectively, with a sensitivity of 63.2% and 80% specificity at 11.59 ng/ml and a sensitivity of 68.4% and 90% specificity at 10.35 ng/ml. At MS, responded NB patients were slightly increased up to 70%. This evaluation was confirmed by further decrease in cfDNA and NSE levels during intermediate chemotherapy in comparison with early stage.
Conclusion: The dynamic change of cfDNA was considered as a surrogate biomarker to evaluate tumor burden and MRD of NB during early and intermediate therapy periods.
Keywords: dynamic changes; minimal residual disease; neuroblastoma; plasma cell free DNA; therapy response.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

