The effects of dynamic compressive loading on human mesenchymal stem cell osteogenesis in the stiff layer of a bilayer hydrogel
- PMID: 30793536
- PMCID: PMC7408337
- DOI: 10.1002/term.2827
The effects of dynamic compressive loading on human mesenchymal stem cell osteogenesis in the stiff layer of a bilayer hydrogel
Abstract
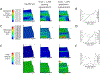
Bilayer hydrogels with a soft cartilage-like layer and a stiff bone-like layer embedded with human mesenchymal stem cells (hMSCs) are promising for osteochondral tissue engineering. The goals of this work were to evaluate the effects of dynamic compressive loading (2.5% applied strain, 1 Hz) on osteogenesis in the stiff layer and spatially map local mechanical responses (strain, stress, hydrostatic pressure, and fluid velocity). A bilayer hydrogel was fabricated from soft (24 kPa) and stiff (124 kPa) poly (ethylene glycol) hydrogels. With hMSCs embedded in the stiff layer, osteogenesis was delayed under loading evident by lower OSX and OPN expressions, alkaline phosphatase activity, and collagen content. At Day 28, mineral deposits were present throughout the stiff layer without loading but localized centrally and near the interface under loading. Local strains mapped by particle tracking showed substantial equivalent strain (~1.5%) transferring to the stiff layer. When hMSCs were cultured in stiff single-layer hydrogels subjected to similar strains, mineralization was inhibited. Finite element analysis revealed that hydrostatic pressures ≥~600 Pa correlated to regions lacking mineralization in both hydrogels. Fluid velocities were low (~1-10 nm/s) in the hydrogels with no apparent correlation to mineralization. Mineralization was recovered by inhibiting ERK1/2, indicating cell-mediated inhibition. These findings suggest that high strains (~1.5%) combined with higher hydrostatic pressures negatively impact osteogenesis, but in a manner that depends on the magnitude of each mechanical response. This work highlights the importance of local mechanical responses in mediating osteogenesis of hMSCs in bilayer hydrogels being studied for osteochondral tissue engineering.
Keywords: bilayer hydrogel; compressive strain; dynamic loading; hydrostatic pressure; mesenchymal stem cells; osteochondral; osteogenesis.
© 2019 John Wiley & Sons, Ltd.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflict of interest.
Figures

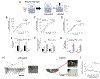

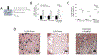

Similar articles
-
Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering.Acta Biomater. 2015 Jul;21:142-53. doi: 10.1016/j.actbio.2015.04.015. Epub 2015 Apr 18. Acta Biomater. 2015. PMID: 25900444
-
Determining Which Hydrostatic Pressure Regimes Promote Osteogenesis in Human Mesenchymal Stem Cells.Tissue Eng Regen Med. 2024 Dec;21(8):1141-1151. doi: 10.1007/s13770-024-00666-w. Epub 2024 Aug 27. Tissue Eng Regen Med. 2024. PMID: 39190133 Free PMC article.
-
Physiological cyclic hydrostatic pressure induces osteogenic lineage commitment of human bone marrow stem cells: a systematic study.Stem Cell Res Ther. 2018 Oct 25;9(1):276. doi: 10.1186/s13287-018-1025-8. Stem Cell Res Ther. 2018. PMID: 30359324 Free PMC article.
-
Mesenchymal Stem Cell Behavior on Soft Hydrogels with Aligned Surface Topographies.ACS Appl Bio Mater. 2022 May 16;5(5):1890-1900. doi: 10.1021/acsabm.1c01260. Epub 2022 Feb 24. ACS Appl Bio Mater. 2022. PMID: 35199983 Review.
-
Recent Advances in Mechanically Loaded Human Mesenchymal Stem Cells for Bone Tissue Engineering.Int J Mol Sci. 2020 Aug 13;21(16):5816. doi: 10.3390/ijms21165816. Int J Mol Sci. 2020. PMID: 32823645 Free PMC article. Review.
Cited by
-
Tissue Engineering Strategies to Increase Osteochondral Regeneration of Stem Cells; a Close Look at Different Modalities.Stem Cell Rev Rep. 2021 Aug;17(4):1294-1311. doi: 10.1007/s12015-021-10130-0. Epub 2021 Feb 5. Stem Cell Rev Rep. 2021. PMID: 33547591 Review.
-
Hydrogel Swelling-Mediated Strain Induces Cell Alignment at Dentin Interfaces.ACS Biomater Sci Eng. 2022 Aug 8;8(8):3568-3575. doi: 10.1021/acsbiomaterials.2c00566. Epub 2022 Jul 6. ACS Biomater Sci Eng. 2022. PMID: 35793542 Free PMC article.
-
Controlled Mechanical Property Gradients Within a Digital Light Processing Printed Hydrogel-Composite Osteochondral Scaffold.Ann Biomed Eng. 2024 Aug;52(8):2162-2177. doi: 10.1007/s10439-024-03516-x. Epub 2024 Apr 29. Ann Biomed Eng. 2024. PMID: 38684606 Free PMC article.
-
3D Mechanical Confinement Directs Muscle Stem Cell Fate and Function.Adv Biol (Weinh). 2025 Apr;9(4):e2400717. doi: 10.1002/adbi.202400717. Epub 2025 Mar 4. Adv Biol (Weinh). 2025. PMID: 40040295 Free PMC article.
-
Pyroptosis in Osteoarthritis: Molecular Mechanisms and Therapeutic Implications.J Inflamm Res. 2024 Feb 8;17:791-803. doi: 10.2147/JIR.S445573. eCollection 2024. J Inflamm Res. 2024. PMID: 38348279 Free PMC article. Review.
References
-
- Armstrong CG, Lai WM, & Mow VC (1984). An analysis of the unconfined compression of articular cartilage. J Biomech Eng, 106(2), 165–173. - PubMed
-
- Bryant SJ, & Anseth KS (2005). Photopolymerization of hydrogel scaffolds. CRC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

