Effect of stent crimping on calcification of transcatheter aortic valves
- PMID: 30793744
- PMCID: PMC6591709
- DOI: 10.1093/icvts/ivz024
Effect of stent crimping on calcification of transcatheter aortic valves
Abstract
Objectives: Although many challenges related to the acute implantation of transcatheter aortic valves have been resolved, durability and early degeneration are currently the main concerns. Recent reports indicate the potential for early valve degeneration and calcification. However, only little is known about the underlying mechanisms behind the early degeneration of these valves. The goal of this study was to test whether stent crimping increases the risk for early calcification.
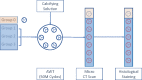
Methods: Stented valves that were crimped at 18-Fr and 14-Fr catheter and uncrimped controls were exposed to a standard calcifying solution for 50 million cycles in an accelerated wear test system. Subsequently, the leaflets of the valves were imaged by microcomputed tomography (micro-CT) followed by histochemical staining and microscopic analyses to quantify calcification and other changes in the leaflets' characteristics.
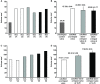
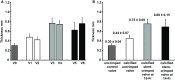
Results: Heavily calcified regions were found over the stent-crimped leaflets compared to uncrimped controls, particularly around the stent's struts. Micro-CT studies measured the total volume of calcification in the uncrimped valves as 77.31 ± 1.63 mm3 vs 95.32 ± 5.20 mm3 in 18-Fr and 110.01 ± 8.33 mm3 in 14-Fr stent-crimped valves, respectively. These results were congruent with the increase in leaflet thickness measured by CT scans (0.44 ± 0.07 mm in uncrimped valves vs 0.69 ± 0.15 mm and 0.75 ± 0.09 mm in 18-Fr and 14-Fr stent-crimped valves, respectively). Histological studies confirmed the micro-CT results, denoting that the percentage of calcification in uncrimped leaflets at the valve's posts was 5.34 ± 3.97 compared to 19.97 ± 6.18 and 27.64 ± 13.17 in the 18-Fr and 14-Fr stent-crimped leaflets, respectively.
Conclusions: This study concludes that stent-crimping damage is associated with a higher level of passive leaflet calcification, which may contribute to early valve degeneration.
Keywords: Calcification; Degeneration; Stent-crimp; Transcatheter aortic valve.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved.
Figures






References
-
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK. et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–20. - PubMed
-
- Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG. et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation 2018;137:388.. - PubMed
-
- Kheradvar A, Groves EM, Goergen CJ, Alavi SH, Tranquillo R, Simmons CA. et al. Emerging trends in heart valve engineering: part II. Novel and standard technologies for aortic valve replacement. Ann Biomed Eng 2015;43:844–57. - PubMed
-
- Zegdi R, Bruneval P, Blanchard D, Fabiani J-N.. Evidence of leaflet injury during percutaneous aortic valve deployment. Eur J Cardiothorac Surg 2011;40:257–60. - PubMed
-
- de Buhr W, Pfeifer S, Slotta-Huspenina J, Wintermantel E, Lutter G, Goetz WA.. Impairment of pericardial leaflet structure from balloon-expanded valved stents. J Thorac Cardiovasc Surg 2012;143:1417–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

