Scaffolding kidney organoids on silk
- PMID: 30793851
- PMCID: PMC6529243
- DOI: 10.1002/term.2830
Scaffolding kidney organoids on silk
Abstract
End stage kidney disease affects hundreds of thousands of patients in the United States. The therapy of choice is kidney replacement, but availability of organs is limited, and alternative sources of tissue are needed. Generation of new kidney tissue in the laboratory has been made possible through pluripotent cell reprogramming and directed differentiation. In current procedures, aggregates of cells known as organoids are grown either submerged or at the air-liquid interface. These studies have demonstrated that kidney tissue can be generated from pluripotent stem cells, but they also identify limitations. The first is that perfusion of cell aggregates is limited, restricting the size to which they can be grown. The second is that aggregates lack the structural integrity required for convenient engraftment and suturing or adhesion to regions of kidney injury. In this study, we evaluated the capacity of silk to serve as a support for the growth and differentiation of kidney tissue from primary cells and from human induced pluripotent stem cells. We find that cells can differentiate to epithelia characteristic of the developing kidney on this material and that these structures are maintained following engraftment under the capsule of the adult kidney. Blood vessel investment can be promoted by the addition of vascular endothelial growth factor to the scaffold, but the proliferation of stromal cells within the graft presents a challenge, which will require some readjustment of cell growth and differentiation conditions. In summary, we find that silk can be used to support growth of stem cell derived kidney tissue.
Keywords: directed differentiation; engraftment; fibroin.
© 2019 John Wiley & Sons, Ltd.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest.
Figures
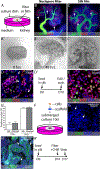

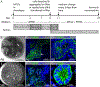
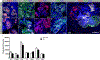
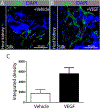

References
-
- Caralt M, Uzarski JS, Iacob S, Obergfell KP, Berg N, Bijonowski BM, Kiefer KM, Ward HH, Wandinger-Ness A, Miller WM, Zhang ZJ, Abecassis MM, Wertheim JA, 2015. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 15, 64–75. - PMC - PubMed
-
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP, 2005. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Developmental cell 9, 283–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

