A Shared Mechanism for the Folding of Voltage-Gated K+ Channels
- PMID: 30793887
- PMCID: PMC6588284
- DOI: 10.1021/acs.biochem.9b00068
A Shared Mechanism for the Folding of Voltage-Gated K+ Channels
Abstract
In this study, we probe the folding of KvAP, a voltage-gated K+ (Kv) channel. The KvAP channel, though of archaebacterial origin, is structurally and functionally similar to eukaryotic Kv channels. An advantage of the KvAP channel is that it can be folded in vitro from an extensively unfolded state and the folding can be controlled by temperature. We utilize these properties of the KvAP channel to separately study the membrane insertion and the tetramerization stages during folding. We use two quantitative assays: a Cys PEGylation assay to monitor membrane insertion and a cross-linking assay to monitor tetramerization. We show that during folding the KvAP polypeptide is rapidly inserted into the lipid bilayer with a "native-like" topology. We identify a segment at the C-terminus that is important for multimerization of the KvAP channel. We show that this C-terminal domain forms a dimer, which raises the possibility that the tetramerization of the KvAP channel proceeds through a dimer of dimers pathway. Our studies show that the in vitro folding of the KvAP channel mirrors aspects of the cellular assembly pathway for voltage-gated K+ channels and therefore suggest that evolutionarily distinct Kv channels share a common folding pathway. The pathway for the folding and assembly of a Kv channel is of central importance as defects in this pathway have been implicated in the etiology of several disease states. Our studies indicate that the KvAP channel provides an experimentally tractable system for elucidating the folding mechanism of Kv channels.
Figures
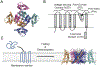







References
-
- Hille B (2001) Ion Channels of Excitable Membranes, Sinauer; Sunderland, MA.
-
- Kuo MM, Haynes WJ, Loukin SH, Kung C, and Saimi Y (2005) Prokaryotic K(+) channels: from crystal structures to diversity, FEMS Microbiol Rev 29, 961–985. - PubMed
-
- Long SB, Tao X, Campbell EB, and MacKinnon R (2007) Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment, Nature 450, 376–382. - PubMed
-
- Pongs O, and Schwarz JR (2010) Ancillary subunits associated with voltage-dependent K+ channels, Physiol Rev 90, 755–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

