Pore Space Partition within a Metal-Organic Framework for Highly Efficient C2H2/CO2 Separation
- PMID: 30793890
- PMCID: PMC11061855
- DOI: 10.1021/jacs.9b00232
Pore Space Partition within a Metal-Organic Framework for Highly Efficient C2H2/CO2 Separation
Abstract
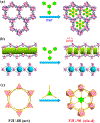
The pore space partition (PSP) approach has been employed to realize a novel porous MOF (FJU-90) with dual functionalities for the challenging C2H2/CO2 separation under ambient conditions. By virtue of a triangular ligand (Tripp = 2,4,6-tris(4-pyridyl)pyridine), the cylindrical channels in the original FJU-88 have been partitioned into uniformly interconnected pore cavities, leading to the dramatically reduced pore apertures from 12.0 × 9.4 to 5.4 × 5.1 Å2. Narrowing down the pore sizes, the resulting activated FJU-90a takes up a very large amount of C2H2 (180 cm3 g-1) but much less of CO2 (103 cm3 g-1) at 298 K and 1 bar, demonstrating it to be the best porous MOF material for this C2H2/CO2 (50%:50%) separation in terms of the C2H2 gravimetric productivity. IAST calculations, molecular modeling studies, and simulated and experimental breakthrough experiments comprehensively demonstrate that the pore space partition strategy is a very powerful approach to constructing MOFs with dual functionality for challenging gas separation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


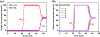
Similar articles
-
Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation.Nat Commun. 2022 Jan 11;13(1):200. doi: 10.1038/s41467-021-27929-7. Nat Commun. 2022. PMID: 35017555 Free PMC article.
-
Integrating the Pillared-Layer Strategy and Pore-Space Partition Method to Construct Multicomponent MOFs for C2H2/CO2 Separation.J Am Chem Soc. 2020 May 20;142(20):9258-9266. doi: 10.1021/jacs.0c00612. Epub 2020 May 7. J Am Chem Soc. 2020. PMID: 32336085
-
A Scalable Pore-space-partitioned Metal-organic Framework Powered by Polycatenation Strategy for Efficient Acetylene Purification.Angew Chem Int Ed Engl. 2025 Feb 3;64(6):e202421992. doi: 10.1002/anie.202421992. Epub 2024 Dec 23. Angew Chem Int Ed Engl. 2025. PMID: 39668752
-
Rational Pore Design of a Cage-like Metal-Organic Framework for Efficient C2H2/CO2 Separation.ACS Appl Mater Interfaces. 2022 Nov 23;14(46):52216-52222. doi: 10.1021/acsami.2c17196. Epub 2022 Nov 10. ACS Appl Mater Interfaces. 2022. PMID: 36356232
-
Optimizing Pore Space for Flexible-Robust Metal-Organic Framework to Boost Trace Acetylene Removal.J Am Chem Soc. 2020 May 27;142(21):9744-9751. doi: 10.1021/jacs.0c02594. Epub 2020 May 14. J Am Chem Soc. 2020. PMID: 32406682
Cited by
-
Spatial disposition of square-planar mononuclear nodes in metal-organic frameworks for C2H2/CO2 separation.Chem Sci. 2022 Oct 17;13(43):12876-12882. doi: 10.1039/d2sc04324f. eCollection 2022 Nov 9. Chem Sci. 2022. PMID: 36519039 Free PMC article.
-
Boosting Adsorption and Selectivity of Acetylene by Nitro Functionalisation in Copper(II)-Based Metal-Organic Frameworks.Angew Chem Int Ed Engl. 2025 Feb 3;64(6):e202417183. doi: 10.1002/anie.202417183. Epub 2024 Dec 27. Angew Chem Int Ed Engl. 2025. PMID: 39627161 Free PMC article.
-
A Chemically Robust Microporous Zn-MOF for C2H2 Separation from CO2 and Industrially Relevant Four Component Gas Mixtures.Small. 2025 Feb;21(8):e2411456. doi: 10.1002/smll.202411456. Epub 2024 Dec 23. Small. 2025. PMID: 39711264 Free PMC article.
-
Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation.Nat Commun. 2022 Jan 11;13(1):200. doi: 10.1038/s41467-021-27929-7. Nat Commun. 2022. PMID: 35017555 Free PMC article.
-
Strengthening Intraframework Interaction within Flexible MOFs Demonstrates Simultaneous Sieving Acetylene from Ethylene and Carbon Dioxide.Adv Sci (Weinh). 2023 Mar;10(9):e2207127. doi: 10.1002/advs.202207127. Epub 2023 Jan 26. Adv Sci (Weinh). 2023. PMID: 36703621 Free PMC article.
References
-
- Furukawa H; Cordova KE; O’Keeffe M; Yaghi OM The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. - PubMed
-
- Li JR; Kuppler RJ; Zhou HC Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477. - PubMed
-
- Bao Z; Chang G; Xing H; Krishna R; Ren Q; Chen B. Potential of microporous metal−organic frameworks for separation of hydrocarbon mixtures. Energy Environ. Sci. 2016, 9, 3612.
-
- Zhao X; Wang Y; Li D-S; Bu X; Feng P. Metal−Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. - PubMed
-
- Cui Y; Li B; He H; Zhou W; Chen B; Qian G. Metal−Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

