The 2018 Chitranjan S. Ranawat, MD Award: Developing and Implementing a Novel Institutional Guideline Strategy Reduced Postoperative Opioid Prescribing After TKA and THA
- PMID: 30794233
- PMCID: PMC6345303
- DOI: 10.1007/s11999.0000000000000292
The 2018 Chitranjan S. Ranawat, MD Award: Developing and Implementing a Novel Institutional Guideline Strategy Reduced Postoperative Opioid Prescribing After TKA and THA
Abstract
Background: Opioid prescription management is challenging for orthopaedic surgeons, and we lack evidence-based guidelines for responsible opioid prescribing. Our institution recently developed opioid prescription guidelines for patients undergoing several common orthopaedic procedures including TKA and THA in an effort to reduce and standardize prescribing patterns.
Questions/purposes: (1) How do opioid prescriptions at discharge and 30-day refill rates change in opioid-naïve patients undergoing primary TKA and THA before and after implementation of a novel prescribing guideline strategy? (2) What patient, surgical, and in-hospital factors influence opioid prescription quantity and refill rate?
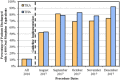
Methods: New institutional guidelines for patients undergoing TKA and THA recommend a maximum postoperative prescription of 400 oral morphine equivalents (OME), comparable to 50 tablets of 5 mg oxycodone or 80 tablets of 50 mg tramadol. All opioid-naïve patients, defined as those who did not take any opioids within 90 days preceding surgery, undergoing primary TKA and THA at a single tertiary care institution were evaluated from program initiation on August 1, 2017, through December 31, 2017, as the postguideline era cohort. This group (n = 751 patients) was compared with all opioid-naïve patients undergoing TKA and THA from 2016 at the same institution (n = 1822 patients). Some providers were early adopters of the guidelines as they were being developed, which is why January to July 2017 was not evaluated. Patients in the preguideline and postguideline eras were not different in terms of age, sex, race, body mass index, education level, employment status, psychiatric illness, marital status, smoking history, outpatient use of benzodiazepines or gabapentinoids, or diagnoses of diabetes mellitus, peripheral neuropathy, or cancer. The primary outcome assessed was adherence to the new guidelines with a secondary outcome of opioid medication refills ordered within 30 days from any provider. Multivariable logistic regression analyses were performed with outcomes of guideline compliance and refills and adjusted for demographic, surgical, and patient care factors. Patients were followed for 30 days after surgery and no patients were lost to followup.
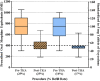
Results: Median opioid prescription and range of prescriptions decreased in the postguideline era compared with the preguideline era (750 OME, interquartile range [IQR] 575-900 OME versus 388 OME, IQR 350-389; difference of medians = 362 OME; p < 0.001). There was no difference among patients undergoing TKA before and after guideline implementation in terms of the 30-day refill rate (35% [349 of 1011] versus 35% [141 of 399]; p = 0.77); this relationship was similar among patient undergoing THA (16% [129 of 811] versus 17% [61 of 352]; p = 0.55). After controlling for relevant patient-level factors, we found that implementation of an institutional guideline was the strongest factor associated with a prescription of ≤ 400 OME (adjusted odds ratio, 36; 95% confidence interval, 25-52; p < 0.001); although a number of patient-level factors also were associated with prescription quantity, the effect sizes were much smaller.
Conclusions: This study provides a proof of concept that institutional guidelines to reduce postoperative opioid prescribing can improve aftercare in patients undergoing arthroplasty in a short period of time. The current report evaluates our experience with the first 5 months of this program; therefore, longer term data will be mandatory to determine longitudinal guideline adherence and whether the cutoffs established by this pilot initiative require further refinement for individual procedures.
Level of evidence: Level II, therapeutic study.
Conflict of interest statement
All ICMJE Conflict of Interest Forms for authors and
Figures
Comment in
-
CORR Insights®: The 2018 Chitranjan S. Ranawat, MD Award: Developing and Implementing a Novel Institutional Guideline Strategy Reduced Postoperative Opioid Prescribing After TKA and THA.Clin Orthop Relat Res. 2019 Jan;477(1):114-115. doi: 10.1097/CORR.0000000000000334. Clin Orthop Relat Res. 2019. PMID: 29698300 Free PMC article. No abstract available.
References
-
- Amundson AW, Johnson RL, Abdel MP, Mantilla CB, Panchamia JK, Taunton MJ, Kralovec ME, Hebl JR, Schroeder DR, Pagnano MW, Kopp SL. A three-arm randomized clinical trial comparing continuous femoral plus single-injection sciatic peripheral nerve blocks versus periarticular injection with ropivacaine or liposomal bupivacaine for patients undergoing total knee arthroplasty. Anesthesiology. 2017;126:1139–1150. - PubMed
-
- Gawande AA. It's time to adopt electronic prescriptions for opioids. Ann Surg. 2017;265:693–694. - PubMed
-
- Hilibrand AS, Matzkin E. Combatting Opioid Misuse. 2017. Available at: https://www.aaos.org/AAOSNow/2017/Jun/YourAAOS/youraaos10/?ssopc=1. Accessed November 18, 2017.
-
- Hill MV, McMahon ML, Stucke RS, Barth RJ., Jr Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

