Association of Prepubertal and Adolescent Androgen Concentrations With Timing of Breast Development and Family History of Breast Cancer
- PMID: 30794303
- PMCID: PMC6484611
- DOI: 10.1001/jamanetworkopen.2019.0083
Association of Prepubertal and Adolescent Androgen Concentrations With Timing of Breast Development and Family History of Breast Cancer
Abstract
Importance: Early breast development is a risk factor for breast cancer, and girls with a breast cancer family history (BCFH) experience breast development earlier than girls without a BCFH.
Objectives: To assess whether prepubertal androgen concentrations are associated with timing of breast development (analysis 1) and to compare serum androgen concentrations in girls with and without a BCFH (analysis 2).
Design, setting, and participants: Prospective cohort study of 104 girls aged 6 to 13 years at baseline using data collected between August 16, 2011, and March 24, 2016, from the Lessons in Epidemiology and Genetics of Adult Cancer From Youth (LEGACY) Girls Study, New York site.
Exposures: Analysis 1 included serum concentrations of dehydroepiandrosterone sulfate, androstenedione, and testosterone (free and total) measured before breast development and divided at the median into high and low categories. Analysis 2 included the degree of BCFH: first-degree was defined as having a mother with breast cancer and second-degree was defined as having a grandmother or aunt with breast cancer.
Main outcomes and measures: Analysis 1 included age at onset of breast development measured using the Pubertal Development Scale (scores range from 1-4; scores ≥2 indicate breast development), and analysis 2 included serum androgen concentrations. We also assessed breast cancer-specific distress using the 8-item Child Impact of Events Scale.
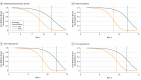
Results: Our analyses included 36 girls for the prospective model, 92 girls for the cross-sectional model, and 104 girls for the longitudinal model. Of the 104 girls, the mean (SD) age at baseline was 10.3 (2.5) years, and 41 (39.4%) were non-Hispanic white, 41 (39.4%) were Hispanic, 13 (12.5%) were non-Hispanic black, and 9 (8.7%) were other race/ethnicity. Forty-two girls (40.4%) had a positive BCFH. Girls with prepubertal androstenedione concentrations above the median began breast development 1.5 years earlier than girls with concentrations below the median (Weibull survival model-estimated median age, 9.4 [95% CI, 9.0-9.8] years vs 10.9 [95% CI, 10.4-11.5] years; P = .001). Similar patterns were observed for dehydroepiandrosterone sulfate (1.1 years earlier: age, 9.6 [95% CI, 9.1-10.1] years vs 10.7 [95% CI, 10.2-11.3] years; P = .009), total testosterone (1.4 years earlier: age, 9.5 [95% CI, 9.1-9.9] years vs 10.9 [95% CI, 10.4-11.5] years; P = .001), and free testosterone (1.1 years earlier: age, 9.7 [95% CI, 9.2-10.1] years vs 10.8 [95% CI, 10.2-11.4] years; P = .01). Compared with girls without BCFH, girls with a first-degree BCFH, but not a second-degree BCFH, had 240% higher androstenedione concentrations (geometric means: no BCFH, 0.49 ng/mL vs first-degree BCFH, 1.8 ng/mL vs second-degree, 1.6 ng/mL; P = .01), 10% higher total testosterone concentrations (12.7 ng/dL vs 14.0 ng/dL vs 13.7 ng/dL; P = .01), and 92% higher free testosterone concentrations (1.3 pg/mL vs 2.5 pg/mL vs 0.3 pg/mL; P = .14). The dehydroepiandrosterone sulfate concentration did not differ between BCFH-positive and BCFH-negative girls but was elevated in girls with breast cancer-specific distress.
Conclusions and relevance: Our findings suggest that androgen concentrations may differ between girls with and without a BCFH and that elevated hormone concentrations during adolescence may be another factor to help explain the familial clustering of breast cancer.
Conflict of interest statement
Figures

Similar articles
-
Influence of pubertal development on urinary oxidative stress biomarkers in adolescent girls in the New York LEGACY cohort.Free Radic Res. 2020 Jun;54(6):431-441. doi: 10.1080/10715762.2020.1798001. Epub 2020 Sep 10. Free Radic Res. 2020. PMID: 32686531 Free PMC article.
-
Pubertal development in girls by breast cancer family history: the LEGACY girls cohort.Breast Cancer Res. 2017 Jun 8;19(1):69. doi: 10.1186/s13058-017-0849-y. Breast Cancer Res. 2017. PMID: 28595647 Free PMC article.
-
Psychosocial Adjustment and Perceived Risk Among Adolescent Girls From Families With BRCA1/2 or Breast Cancer History.J Clin Oncol. 2016 Oct 1;34(28):3409-16. doi: 10.1200/JCO.2015.66.3450. Epub 2016 Aug 22. J Clin Oncol. 2016. PMID: 27551110 Free PMC article.
-
Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis.JAMA Pediatr. 2020 Apr 1;174(4):e195881. doi: 10.1001/jamapediatrics.2019.5881. Epub 2020 Apr 6. JAMA Pediatr. 2020. PMID: 32040143 Free PMC article.
-
Identifying opportunities for cancer prevention during preadolescence and adolescence: puberty as a window of susceptibility.J Adolesc Health. 2013 May;52(5 Suppl):S15-20. doi: 10.1016/j.jadohealth.2012.09.019. J Adolesc Health. 2013. PMID: 23601607 Free PMC article. Review.
Cited by
-
CYP11B1 variants influence skeletal maturation via alternative splicing.Commun Biol. 2021 Nov 9;4(1):1274. doi: 10.1038/s42003-021-02774-y. Commun Biol. 2021. PMID: 34754074 Free PMC article.
-
Immigrant generation status and its association with pubertal timing and tempo among Hispanic girls and boys.Am J Hum Biol. 2023 Oct;35(10):e23940. doi: 10.1002/ajhb.23940. Epub 2023 Jun 20. Am J Hum Biol. 2023. PMID: 37338197 Free PMC article.
-
Influence of pubertal development on urinary oxidative stress biomarkers in adolescent girls in the New York LEGACY cohort.Free Radic Res. 2020 Jun;54(6):431-441. doi: 10.1080/10715762.2020.1798001. Epub 2020 Sep 10. Free Radic Res. 2020. PMID: 32686531 Free PMC article.
-
The Other Side of the Coin: May Androgens Have a Role in Breast Cancer Risk?Int J Mol Sci. 2021 Dec 31;23(1):424. doi: 10.3390/ijms23010424. Int J Mol Sci. 2021. PMID: 35008851 Free PMC article. Review.
-
Urinary Androgens Provide Additional Evidence Related to Metabolism and Are Correlated With Serum Androgens in Girls.J Endocr Soc. 2024 Jan 16;8(3):bvad161. doi: 10.1210/jendso/bvad161. eCollection 2024 Jan 16. J Endocr Soc. 2024. PMID: 38234314 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

