Knockout of receptor for advanced glycation end-products attenuates age-related renal lesions
- PMID: 30794349
- PMCID: PMC6413655
- DOI: 10.1111/acel.12850
Knockout of receptor for advanced glycation end-products attenuates age-related renal lesions
Abstract
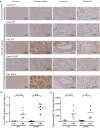
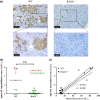
Pro-aging effects of endogenous advanced glycation end-products (AGEs) have been reported, and there is increasing interest in the pro-inflammatory and -fibrotic effects of their binding to RAGE (the main AGE receptor). The role of dietary AGEs in aging remains ill-defined, but the predominantly renal accumulation of dietary carboxymethyllysine (CML) suggests the kidneys may be particularly affected. We studied the impact of RAGE invalidation and a CML-enriched diet on renal aging. Two-month-old male, wild-type (WT) and RAGE-/- C57Bl/6 mice were fed a control or a CML-enriched diet (200 μg CML/gfood ) for 18 months. Compared to controls, we observed higher CML levels in the kidneys of both CML WT and CML RAGE-/- mice, with a predominantly tubular localization. The CML-rich diet had no significant impact on the studied renal parameters, whereby only a trend to worsening glomerular sclerosis was detected. Irrespective of diet, RAGE-/- mice were significantly protected against nephrosclerosis lesions (hyalinosis, tubular atrophy, fibrosis and glomerular sclerosis) and renal senile apolipoprotein A-II (ApoA-II) amyloidosis (p < 0.001). A positive linear correlation between sclerosis score and ApoA-II amyloidosis score (r = 0.92) was observed. Compared with old WT mice, old RAGE-/- mice exhibited lower expression of inflammation markers and activation of AKT, and greater expression of Sod2 and SIRT1. Overall, nephrosclerosis lesions and senile amyloidosis were significantly reduced in RAGE-/- mice, indicating a protective effect of RAGE deletion with respect to renal aging. This could be due to reduced inflammation and oxidative stress in RAGE-/- mice, suggesting RAGE is an important receptor in so-called inflamm-aging.
Keywords: advanced glycation end-products; amyloidosis; chronic kidney disease; nephrosclerosis; receptor for AGEs; renal aging.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Alderson, H. V. , Ritchie, J. P. , Pagano, S. , Middleton, R. J. , Pruijm, M. , Vuilleumier, N. , & Kalra, P. A. (2016). The associations of blood kidney injury molecule‐1 and neutrophil gelatinase‐associated lipocalin with progression from CKD to ESRD. Clinical Journal of the American Society of Nephrology, 11(12), 2141–2149. 10.2215/CJN.02670316 - DOI - PMC - PubMed
-
- Anantharamaiah, G. M. , Hughes, T. A. , Iqbal, M. , Gawish, A. , Neame, P. J. , Medley, M. F. , & Segrest, J. P. (1988). Effect of oxidation on the properties of apolipoproteins A‐I and A‐II. Journal of Lipid Research, 29(3), 309–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

