Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: sex differences in healthy young adults
- PMID: 30794437
- PMCID: PMC6589599
- DOI: 10.1152/ajpregu.00305.2018
Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: sex differences in healthy young adults
Abstract
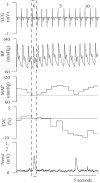
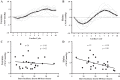
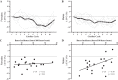
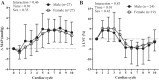
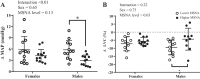
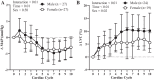
Previous studies have demonstrated an inverse relation between resting muscle sympathetic nerve activity (MSNA) and vasoconstrictor responsiveness (i.e., sympathetic transduction), such that those with high resting MSNA have low vascular responsiveness, and vice versa. The purpose of this investigation was to determine whether biological sex influences the balance between resting MSNA and beat-to-beat sympathetic transduction. We measured blood pressure (BP) and MSNA during supine rest in 54 healthy young adults (27 females: 23 ± 4 yr, 107 ± 8/63 ± 8 mmHg; 27 males: 25 ± 3 yr, 115 ± 11/64 ± 7 mmHg; means ± SD). We quantified beat-to-beat fluctuations in mean arterial pressure (MAP, mmHg) and limb vascular conductance (LVC, %) for 10 cardiac cycles after each MSNA burst using signal averaging, an index of sympathetic vascular transduction. In females, there was no correlation between resting MSNA (burst incidence; burst/100 heartbeats) and peak ΔMAP (r = -0.10, P = 0.62) or peak ΔLVC (r = -0.12, P = 0.63). In males, MSNA was related to peak ΔMAP (r = -0.50, P = 0.01) and peak ΔLVC (r = 0.49, P = 0.03); those with higher resting MSNA had blunted increases in MAP and reductions in LVC in response to a burst of MSNA. In a sub-analysis, we performed a median split between high- versus low-MSNA status on ΔMAP and ΔLVC within each sex and found that only males demonstrated a significant difference in ΔMAP and ΔLVC between high- versus low-MSNA groups. These findings support an inverse relation between resting MSNA and sympathetic vascular transduction in males only and advance our understanding on the influence of biological sex on sympathetic nervous system-mediated alterations in beat-to-beat BP regulation.
Keywords: blood pressure; muscle sympathetic nerve activity; sex differences; vascular physiology; vasoconstriction.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Brian MS, Matthews EL, Watso JC, Babcock MC, Wenner MM, Rose WC, Stocker SD, Farquhar WB. The influence of acute elevations in plasma osmolality and serum sodium on sympathetic outflow and blood pressure responses to exercise. J Neurophysiol 119: 1257–1265, 2018. doi:10.1152/jn.00559.2017. - DOI - PMC - PubMed
-
- Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572: 821–827, 2006. doi:10.1113/jphysiol.2005.104075. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

