Routine surveillance for the identification of drug resistant Tuberculosis in Tanzania: A cross-sectional study of stakeholders' perceptions
- PMID: 30794620
- PMCID: PMC6386308
- DOI: 10.1371/journal.pone.0212421
Routine surveillance for the identification of drug resistant Tuberculosis in Tanzania: A cross-sectional study of stakeholders' perceptions
Abstract
Background: Routine surveillance is required to monitor the performance of tuberculosis diagnostic programme and is essential for the rapid detection of drug resistance. The main objective of this study was to explore the effectiveness and stakeholder perception of the current routine surveillance system for previously treated tuberculosis cases in Tanzania with a view to identify interventions to improve and accelerate positive patient outcomes.
Methods: A study using quantitative and qualitative methods of data collection including in-depth interviews and focus group discussions with health care service providers was conducted in four regions. Quantitative data were extracted from the routine databases to assess performance.
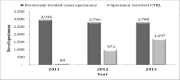
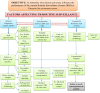
Results: Quantitative findings from 2011 to 2013 showed 2,750 specimens from previously treated TB cases were received at the reference laboratory. The number increased year on year, but even in the most recent year was only 61% of that expected. The median and interquartile range of turnaround time in days from specimen reception to results reported for smear microscopy, culture and drug susceptibility testing were 1(1, 1), 61(43, 71) and 129(72, 170) respectively. Contaminated specimens were reported in 3.6% of cases. The qualitative analysis showed the system of sending specimens using postal services was seen to be efficient by participants. However, there were many challenges and significant delays in specimens reaching the reference laboratory associated with lack of funds to transfer specimens, weak form completion, inadequate training and poor supervision. These all adversely affected the implementation of the routine surveillance system.
Conclusions: Many issues limit the effectiveness of the routine surveillance system in Tanzania. Priority areas for strengthening are; specimen transportation, supervision and availability of commodities. A pilot study of a revised routine surveillance system that takes into account the observations from this study alongside improved access to drug susceptibility testing using Xpert MTB/RIF should be considered.
Conflict of interest statement
The authors have declared that no competing interests exists.
Figures
References
-
- WHO. World Health Organisation Tuberculosis Global Report 2016.
-
- Acosta CD, Rusovich V, Harries AD, Ahmedov S, van den Boom M, Dara M, et al. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. Int J Tuberc Lung Dis. 2014;2: 1–2. 10.1136/bmj.h111 - DOI
-
- WHO | WHO endorses new rapid tuberculosis test WHO. World Health Organization; 2010. Available: http://www.who.int/mediacentre/news/releases/2010/tb_test_20101208/en/#....
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources