Widespread insecticide resistance in Aedes aegypti L. from New Mexico, U.S.A
- PMID: 30794644
- PMCID: PMC6386485
- DOI: 10.1371/journal.pone.0212693
Widespread insecticide resistance in Aedes aegypti L. from New Mexico, U.S.A
Abstract
Background: Aedes aegypti mosquitoes are vectors of a variety of emerging viral pathogens, including yellow fever, dengue, chikungunya, and Zika virus. This species has established endemic populations in all cities across southern New Mexico sampled to date. Presently, control of Aedes-borne viruses relies on deployment of insecticides to suppress mosquito populations, but the evolution of insecticide resistance threatens the success of vector control programs. While insecticide resistance is quite common in Ae. aegypti field populations across much of the U.S., the resistance status of this species in populations from New Mexico has not previously been assessed.
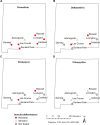
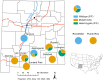
Results: First, we collected information on pesticide use in cities in southern New Mexico and found that the most commonly used active ingredients were pyrethroids. The use of insecticides with the same mode-of-action over multiple years is likely to promote the evolution of resistance. To determine if there was evidence of resistance in some cities in southern New Mexico, we collected Ae. aegypti from the same cities and established laboratory strains to assess resistance to pyrethroid insecticides and, for a subset of populations, to organophosphate insecticides. F2 or F4 generation mosquitoes were assessed for insecticide resistance using bottle test bioassays. The majority of the populations from New Mexico that we analyzed were resistant to the pyrethroids permethrin and deltamethrin. A notable exception to this trend were mosquitoes from Alamogordo, a city that did not report using pyrethroid insecticides for vector control. We screened individuals from each population for known knock down resistance (kdr) mutations via PCR and found a strong association between the presences of the F1534C kdr mutation in the para gene of Ae. aegypti (homologue to F1534C in Musca domestica L.) and pyrethroid resistance.
Conclusion: High-level pyrethroid resistance is common in Ae. aegypti from New Mexico and geographic variation in such resistance is likely associated with variation in usage of pyrethroids for vector control. Resistance monitoring and management is recommended in light of the potential for arbovirus outbreaks in this state. Also, alternative approaches to mosquito control that do not involve insecticides should be explored.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Clements AN, editor. The Biology of Mosquitoes. London: Chapman & Hall; 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

