ASB20123: A novel C-type natriuretic peptide derivative for treatment of growth failure and dwarfism
- PMID: 30794654
- PMCID: PMC6386482
- DOI: 10.1371/journal.pone.0212680
ASB20123: A novel C-type natriuretic peptide derivative for treatment of growth failure and dwarfism
Abstract
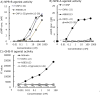
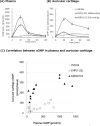
C-type natriuretic peptide (CNP) and its receptor natriuretic peptide receptor B (NPR-B) are physiological potent positive regulators of endochondral bone growth; therefore, the CNP/NPR-B signaling pathway is one of the most promising therapeutic targets for treating growth failure and dwarfism. In this article, we summarized the pharmacological properties of a novel CNP analog peptide ASB20123 as a therapeutic agent for short stature. ASB20123, one of the CNP/ghrelin chimeric peptides, is composed of CNP(1-22) and human ghrelin(12-28, E17D). Compared to CNP(1-22), ASB20123 showed similar agonist activity for NPR-B and improved biokinetics with a longer plasma half-life in rats. In addition, the distribution of ASB20123 to the cartilage was higher than that of CNP(1-22) after single subcutaneous (sc) injection to mice. These results suggested that the C-terminal part of ghrelin, which has clusters of basic amino acid residues and a BX7B motif, might contribute to the retention of ASB20123 in the extracellular matrix of the growth plate. Multiple sc doses of ASB20123 potently stimulated skeletal growth in rats in a dose-dependent manner, and sc infusion was more effective than bolus injection at the same dose. Our data indicated that high plasma levels of ASB20123 would not necessarily be required for bone growth acceleration. Thus, pharmaceutical formulation approaches for sustained-release dosage forms to allow chronic exposure to ASB20123 might be suitable to ensure drug effectiveness and safety.
Conflict of interest statement
KK has nothing to disclose. NM, TY, AY, KY, SY, RN, TJ, MF, HM, and YM are employed by Asubio pharma Co., Ltd and/or Daiichi Sankyo Co., Ltd. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures





Similar articles
-
Safety assessment of a novel C-type natriuretic peptide derivative and the mechanism of bone- and cartilage-specific toxicity.PLoS One. 2019 Sep 11;14(9):e0218229. doi: 10.1371/journal.pone.0218229. eCollection 2019. PLoS One. 2019. PMID: 31509532 Free PMC article.
-
Design and evaluation of novel natriuretic peptide derivatives with improved pharmacokinetic and pharmacodynamic properties.Peptides. 2017 Nov;97:16-21. doi: 10.1016/j.peptides.2017.09.008. Epub 2017 Sep 9. Peptides. 2017. PMID: 28899838
-
Neprilysin Inhibition Promotes Skeletal Growth via the CNP/NPR-B Pathway.Endocrinology. 2024 May 27;165(7):bqae058. doi: 10.1210/endocr/bqae058. Endocrinology. 2024. PMID: 38752331
-
Role of C-type natriuretic peptide signalling in maintaining cartilage and bone function.Osteoarthritis Cartilage. 2014 Nov;22(11):1800-7. doi: 10.1016/j.joca.2014.07.018. Epub 2014 Jul 31. Osteoarthritis Cartilage. 2014. PMID: 25086404 Review.
-
C-type natriuretic peptide in growth: a new paradigm.Growth Horm IGF Res. 2006 Jul;16 Suppl A:S6-14. doi: 10.1016/j.ghir.2006.03.016. Epub 2006 May 22. Growth Horm IGF Res. 2006. PMID: 16716628 Review.
Cited by
-
Advances in the mechanism and therapies of achondroplasia.Genes Dis. 2024 Sep 24;12(4):101436. doi: 10.1016/j.gendis.2024.101436. eCollection 2025 Jul. Genes Dis. 2024. PMID: 40256430 Free PMC article. Review.
-
A long-acting C-natriuretic peptide for achondroplasia.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2201067119. doi: 10.1073/pnas.2201067119. Epub 2022 Jul 18. Proc Natl Acad Sci U S A. 2022. PMID: 35858423 Free PMC article.
-
Safety assessment of a novel C-type natriuretic peptide derivative and the mechanism of bone- and cartilage-specific toxicity.PLoS One. 2019 Sep 11;14(9):e0218229. doi: 10.1371/journal.pone.0218229. eCollection 2019. PLoS One. 2019. PMID: 31509532 Free PMC article.
-
Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours.Int J Mol Sci. 2021 Jan 22;22(3):1076. doi: 10.3390/ijms22031076. Int J Mol Sci. 2021. PMID: 33499110 Free PMC article.
-
Natriuretic Peptide-Based Novel Therapeutics: Long Journeys of Drug Developments Optimized for Disease States.Biology (Basel). 2022 Jun 3;11(6):859. doi: 10.3390/biology11060859. Biology (Basel). 2022. PMID: 35741380 Free PMC article. Review.
References
-
- Ogawa Y, Itoh H, Nakao K. Molecular biology and biochemistry of natriuretic peptide family. Clin Exp Pharmacol Physiol. 1995; 22(1):49–53. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

