β-Catenin and Yes-Associated Protein 1 Cooperate in Hepatoblastoma Pathogenesis
- PMID: 30794807
- PMCID: PMC6521893
- DOI: 10.1016/j.ajpath.2019.02.002
β-Catenin and Yes-Associated Protein 1 Cooperate in Hepatoblastoma Pathogenesis
Erratum in
-
Correction.Am J Pathol. 2019 Aug;189(8):1680. doi: 10.1016/j.ajpath.2019.06.003. Am J Pathol. 2019. PMID: 31345468 Free PMC article. No abstract available.
Abstract
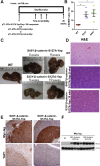
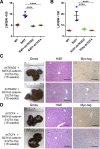
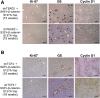
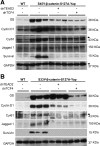
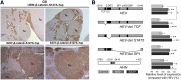
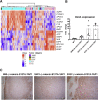
Hepatoblastoma (HB), the most common pediatric primary liver neoplasm, shows nuclear localization of β-catenin and yes-associated protein 1 (YAP1) in almost 80% of the cases. Co-expression of constitutively active S127A-YAP1 and ΔN90 deletion-mutant β-catenin (YAP1-ΔN90-β-catenin) causes HB in mice. Because heterogeneity in downstream signaling is being identified owing to mutational differences even in the β-catenin gene alone, we investigated if co-expression of point mutants of β-catenin (S33Y or S45Y) with S127A-YAP1 led to similar tumors as YAP1-ΔN90-β-catenin. Co-expression of S33Y/S45Y-β-catenin and S127A-YAP1 led to activation of Yap and Wnt signaling and development of HB, with 100% mortality by 13 to 14 weeks. Co-expression with YAP1-S45Y/S33Y-β-catenin of the dominant-negative T-cell factor 4 or dominant-negative transcriptional enhanced associate domain 2, the respective surrogate transcription factors, prevented HB development. Although histologically similar, HB in YAP1-S45Y/S33Y-β-catenin, unlike YAP1-ΔN90-β-catenin HB, was glutamine synthetase (GS) positive. However, both ΔN90-β-catenin and point-mutant β-catenin comparably induced GS-luciferase reporter in vitro. Finally, using a previously reported 16-gene signature, it was shown that YAP1-ΔN90-β-catenin HB tumors exhibited genetic similarities with more proliferative, less differentiated, GS-negative HB patient tumors, whereas YAP1-S33Y/S45Y-β-catenin HB exhibited heterogeneity and clustered with both well-differentiated GS-positive and proliferative GS-negative patient tumors. Thus, we demonstrate that β-catenin point mutants can also collaborate with YAP1 in HB development, albeit with a distinct molecular profile from the deletion mutant, which may have implications in both biology and therapy.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Czauderna P., Haeberle B., Hiyama E., Rangaswami A., Krailo M., Maibach R., Rinaldi E., Feng Y., Aronson D., Malogolowkin M., Yoshimura K., Leuschner I., Lopez-Terrada D., Hishiki T., Perilongo G., von Schweinitz D., Schmid I., Watanabe K., Derosa M., Meyers R. The Children's Hepatic tumors International Collaboration (CHIC): novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer. 2016;52:92–101. - PMC - PubMed
-
- Perilongo G., Malogolowkin M., Feusner J. Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer. 2012;59:818–821. - PubMed
-
- Czauderna P., Lopez-Terrada D., Hiyama E., Haberle B., Malogolowkin M.H., Meyers R.L. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014;26:19–28. - PubMed
-
- Koch A., Denkhaus D., Albrecht S., Leuschner I., von Schweinitz D., Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999;59:269–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

