Genetic Strain and Sex Differences in a Hyperoxia-Induced Mouse Model of Varying Severity of Bronchopulmonary Dysplasia
- PMID: 30794808
- PMCID: PMC6526502
- DOI: 10.1016/j.ajpath.2019.01.014
Genetic Strain and Sex Differences in a Hyperoxia-Induced Mouse Model of Varying Severity of Bronchopulmonary Dysplasia
Abstract
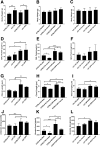
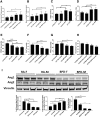
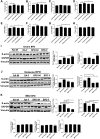
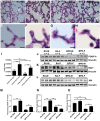
Bronchopulmonary dysplasia (BPD) is a disease prevalent in preterm babies with a need for supplemental oxygen, resulting in impaired lung development and dysregulated vascularization. Epidemiologic studies have shown that males are more prone to BPD and have a delayed recovery compared with females, for reasons unknown. Herein, we tried to recapitulate mild, moderate, and severe BPD, using two different strains of mice, in males and females: CD1 (outbred) and C57BL/6 (inbred). Aside from higher body weight in the CD1 strain, there were no other gross morphologic differences with respect to alveolar development between the two strains. With respect to lung morphology after oxygen exposure, females had less injury with better preservation of alveolar chord length and decreased alveolar protein leak and inflammatory cells in the bronchoalveolar lavage fluid. In addition, housekeeping genes, which are routinely used as loading controls, were expressed differently in males and females. In the BPD mouse model, gonadotropin-releasing hormone was increased in females compared with males. Specific miRNAs (miR-146 and miR-34a) were expressed differently in the sexes. In the severe BPD mouse model, administering miR-146 mimic to males attenuated lung damage, whereas administering miR-146 inhibitor to females increased pulmonary injury.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Sigmund C.D. Viewpoint: are studies in genetically altered mice out of control? Arterioscler Thromb Vasc Biol. 2000;20:1425–1429. - PubMed
-
- Schadt E.E., Monks S.A., Drake T.A., Lusis A.J., Che N., Colinayo V., Ruff T.G., Milligan S.B., Lamb J.R., Cavet G., Linsley P.S., Mao M., Stoughton R.B., Friend S.H. Genetics of gene expression surveyed in maize, mouse and man. Nature. 2003;422:297–302. - PubMed
-
- Chia R., Achilli F., Festing M.F.W., Fisher E.M.C. The origins and uses of mouse outbred stocks. Nat Genet. 2005;37:1181–1186. - PubMed
-
- Johnston C.J., Stripp B.R., Piedbeouf B., Wright T.W., Mango G.W., Reed C.K., Finkelstein J.N. Inflammatory and epithelial responses in mouse strains that differ in sensitivity to hyperoxic injury. Exp Lung Res. 1998;24:189–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

