A case study on the application of an expert-driven read-across approach in support of quantitative risk assessment of p,p'-dichlorodiphenyldichloroethane
- PMID: 30794837
- PMCID: PMC6854443
- DOI: 10.1016/j.yrtph.2019.02.010
A case study on the application of an expert-driven read-across approach in support of quantitative risk assessment of p,p'-dichlorodiphenyldichloroethane
Abstract
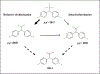
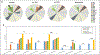
Deriving human health risk estimates for environmental chemicals has traditionally relied on in vivo toxicity databases to characterize potential adverse health effects and associated dose-response relationships. In the absence of in vivo toxicity information, new approach methods (NAMs) such as read-across have the potential to fill the required data gaps. This case study applied an expert-driven read-across approach to identify and evaluate analogues to fill non-cancer oral toxicity data gaps for p,p'-dichlorodiphenyldichloroethane (p,p'-DDD), an organochlorine contaminant known to occur at contaminated sites in the U.S. The source analogue p,p'-dichlorodiphenyltrichloroethane (DDT) and its no-observed-adverse-effect level of 0.05 mg/kg-day were proposed for the derivation of screening-level health reference values for the target chemical, p,p'-DDD. Among the primary similarity contexts (structure, toxicokinetics, and toxicodynamics), toxicokinetic considerations were instrumental in separating p,p'-DDT as the best source analogue from other potential candidates (p,p'-DDE and methoxychlor). In vitro high-throughput screening (HTS) assays from ToxCast were used to evaluate similarity in bioactivity profiles and make inferences toward plausible mechanisms of toxicity to build confidence in the read-across approach. This work demonstrated the value of NAMs such as read-across and in vitro HTS in human health risk assessment of environmental contaminants with the potential to inform regulatory decision-making.
Keywords: In vitro high-throughput screening; Quantitative risk assessment; Read-across; Toxicokinetics.
Published by Elsevier Inc.
Figures
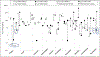

References
-
- Al-Saleh I, et al. , 2012. Levels of DDT and its metabolites in placenta, maternal and cord blood and their potential influence on neonatal anthropometric measures. Sci Total Environ. 416, 62–74. - PubMed
-
- Androutsopoulos VP, et al. , 2013. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology. 307, 89–94. - PubMed
-
- Arnold SF, et al. , 1996. Differential interaction of natural and synthetic estrogens with extracellular binding proteins in a yeast estrogen screen. Steroids. 61, 642–6. - PubMed
-
- ATSDR, 2002a. Toxicological Profile for DDT, DDE, and DDD. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. - PubMed
-
- ATSDR, 2002b. Toxicological Profile for Methoxychlor. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

