Ethanol-induced conditioned place preference and aversion differentially alter plasticity in the bed nucleus of stria terminalis
- PMID: 30795004
- PMCID: PMC6785142
- DOI: 10.1038/s41386-019-0349-0
Ethanol-induced conditioned place preference and aversion differentially alter plasticity in the bed nucleus of stria terminalis
Abstract
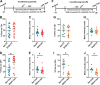
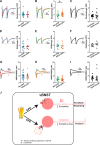
Contextual cues associated with drugs of abuse, such as ethanol, can trigger craving and drug-seeking behavior. Pavlovian procedures, such as place conditioning, have been widely used to study the rewarding/aversive properties of drugs and the association between environmental cues and drug seeking. Previous research has shown that ethanol as an unconditioned stimulus can induce a strong conditioned place preference (CPP) or aversion (CPA) in rodents. However, the neural mechanisms underlying ethanol-induced reward and aversion have not been thoroughly investigated. The bed nucleus of the stria terminalis (BNST), an integral part of the extended amygdala, is engaged by both rewarding and aversive stimuli and plays a role in ethanol-seeking behavior. Here, we used ex-vivo slice physiology to probe learning-induced synaptic plasticity in the BNST following ethanol-induced CPP and CPA. Male DBA/2 J mice (2-3 months old) were conditioned using previously reported ethanol-induced CPP/CPA procedures. Ethanol-induced CPP was associated with increased neuronal excitability in the ventral BNST (vBNST). Conversely, ethanol-induced CPA resulted in a significant decrease in spontaneous glutamatergic transmission without alterations in GABAergic signaling. Ethanol-CPA also led to a significant increase in the paired-pulse ratio at excitatory synapses, suggestive of a decrease in presynaptic glutamate release. Collectively, these data demonstrate that the vBNST is involved in the modulation of contextual learning associated with both the rewarding and the aversive properties of ethanol in mice.
Figures



Comment in
-
The BNST balances alcohol's aversive and rewarding properties.Neuropsychopharmacology. 2019 Oct;44(11):1839-1840. doi: 10.1038/s41386-019-0374-z. Epub 2019 Apr 1. Neuropsychopharmacology. 2019. PMID: 30932025 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

