L-765,314 Suppresses Melanin Synthesis by Regulating Tyrosinase Activity
- PMID: 30795539
- PMCID: PMC6412649
- DOI: 10.3390/molecules24040773
L-765,314 Suppresses Melanin Synthesis by Regulating Tyrosinase Activity
Abstract
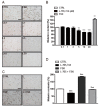
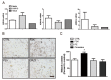
Although melanin production is a key self-defense mechanism against ultraviolet radiation (UVR)-induced skin damage, uneven or excessive deposition of melanin causes hyperpigmentary disorders. Currently available whitening agents are unsatisfactory because of issues with efficacy and safety. To develop more effective depigmenting agents, we performed high-throughput melanin content assay screening using the B16F10 melanoma cell line and identified L-765,314 as a drug that suppressed melanin production in cultured melanocytes in a dose-dependent manner as well as cAMP- or 12-O-tetradecanoylphorbol 13-acetate (TPA)-stimulated melanin production without cytotoxicity. Interestingly, melanogenic gene expression was not altered by L-765,314. Rather, diminished melanin production by L-765,314 appeared to be caused by downregulation of tyrosinase activity via inhibition of protein kinase C (PKC). Because L-765,314 did not show any adverse effect in melanocytes, altogether our data suggest that L-765,314 could be a potential therapeutic candidate for skin hyperpigmentary disorders and further discovery of selective inhibitors targeting PKC might be a promising strategy for the development of depigmenting agents to treat hyperpigmentary disorders.
Keywords: L-765,314; depigmenting agents; protein kinase C; tyrosinase activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Partially purified components of Nardostachys chinensis suppress melanin synthesis through ERK and Akt signaling pathway with cAMP down-regulation in B16F10 cells.J Ethnopharmacol. 2011 Oct 11;137(3):1207-14. doi: 10.1016/j.jep.2011.07.047. Epub 2011 Jul 26. J Ethnopharmacol. 2011. PMID: 21816215
-
Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells.J Ethnopharmacol. 2011 Oct 11;137(3):1183-8. doi: 10.1016/j.jep.2011.07.050. Epub 2011 Jul 26. J Ethnopharmacol. 2011. PMID: 21810462
-
Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2,4 dihydroxybenzylidene)pyrrolidine-2,5-dione (3-DBP).Biochim Biophys Acta. 2012 Jul;1820(7):962-9. doi: 10.1016/j.bbagen.2012.03.018. Epub 2012 Apr 3. Biochim Biophys Acta. 2012. PMID: 22498140
-
Small-Molecule Tyrosinase Inhibitors for Treatment of Hyperpigmentation.Molecules. 2025 Feb 8;30(4):788. doi: 10.3390/molecules30040788. Molecules. 2025. PMID: 40005101 Free PMC article. Review.
-
Natural Tyrosinase Inhibitors: Role of Herbals in the Treatment of Hyperpigmentary Disorders.Mini Rev Med Chem. 2019;19(10):796-808. doi: 10.2174/1389557519666190116101039. Mini Rev Med Chem. 2019. PMID: 31244414 Review.
Cited by
-
Anti-melanogenic property of ginsenoside Rf from Panax ginseng via inhibition of CREB/MITF pathway in melanocytes and ex vivo human skin.J Ginseng Res. 2021 Sep;45(5):555-564. doi: 10.1016/j.jgr.2020.11.003. Epub 2020 Dec 9. J Ginseng Res. 2021. PMID: 34803425 Free PMC article.
-
FGIN-1-27 Inhibits Melanogenesis by Regulating Protein Kinase A/cAMP-Responsive Element-Binding, Protein Kinase C-β, and Mitogen-Activated Protein Kinase Pathways.Front Pharmacol. 2020 Dec 3;11:602889. doi: 10.3389/fphar.2020.602889. eCollection 2020. Front Pharmacol. 2020. PMID: 33390991 Free PMC article.
-
JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression.Theranostics. 2020 Mar 4;10(9):4017-4029. doi: 10.7150/thno.41502. eCollection 2020. Theranostics. 2020. PMID: 32226536 Free PMC article.
References
-
- Nagata H., Takekoshi S., Takeyama R., Homma T., Yoshiyuki Osamura R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004;17:66–73. doi: 10.1046/j.1600-0749.2003.00113.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

