A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016
- PMID: 30795596
- PMCID: PMC6412313
- DOI: 10.3390/molecules24040781
A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016
Abstract
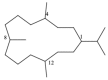
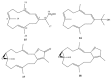
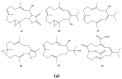
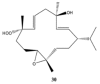
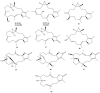
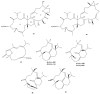
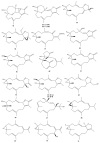
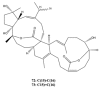
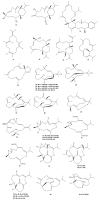
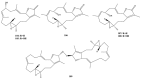
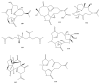
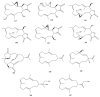
This work reviews the new isolated cembranoid derivatives from species of the genera Sarcophyton, Sinularia, and Lobophytum as well as their biological properties, during 2016⁻2018. The compilation permitted to conclude that much more new cembranoid diterpenes were found in the soft corals of the genus Sarcophyton than in those belonging to the genera Lobophytum or Sinularia. Beyond the chemical composition, the biological properties were also reviewed, namely anti-microbial against several Gram-positive and Gram-negative bacteria and fungi, anti-inflammatory and anti-tumoral against several types of cancer cells. In spite of the biological activities detected in almost all samples, there is a remarkable diversity in the results which may be attributed to the chemical variability that needs to be deepened in order to develop new molecules with potential application in medicine.
Keywords: Lobophytum; Sarcophyton; Sinularia; anti-inflammatory; anti-microbial; anti-tumoral.
Conflict of interest statement
There are no conflicts to declare.
Figures

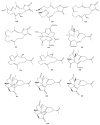
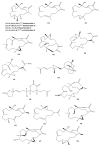
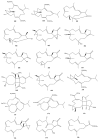

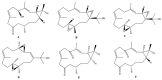
Similar articles
-
Cembranoids from the soft corals Sinularia granosa and Sinularia querciformis.Chem Pharm Bull (Tokyo). 2010 Apr;58(4):464-6. doi: 10.1248/cpb.58.464. Chem Pharm Bull (Tokyo). 2010. PMID: 20410624
-
Cembranoid-Related Metabolites and Biological Activities from the Soft Coral Sinularia flexibilis.Mar Drugs. 2018 Aug 9;16(8):278. doi: 10.3390/md16080278. Mar Drugs. 2018. PMID: 30096866 Free PMC article.
-
Terpenes from the soft corals of the genus Sarcophyton: chemistry and biological activities.Chem Biodivers. 2013 Dec;10(12):2161-96. doi: 10.1002/cbdv.201200122. Chem Biodivers. 2013. PMID: 24327439 Review.
-
Cytotoxic Effects of Sarcophyton sp. Soft Corals-Is There a Correlation to Their NMR Fingerprints?Mar Drugs. 2017 Jul 4;15(7):211. doi: 10.3390/md15070211. Mar Drugs. 2017. PMID: 28677625 Free PMC article.
-
Cembranoids of Soft Corals: Recent Updates and Their Biological Activities.Nat Prod Bioprospect. 2021 Jun;11(3):243-306. doi: 10.1007/s13659-021-00303-2. Epub 2021 Apr 22. Nat Prod Bioprospect. 2021. PMID: 33890249 Free PMC article. Review.
Cited by
-
Lobosteroids A-F: Six New Highly Oxidized Steroids from the Chinese Soft Coral Lobophytum sp.Mar Drugs. 2023 Aug 19;21(8):457. doi: 10.3390/md21080457. Mar Drugs. 2023. PMID: 37623738 Free PMC article.
-
Polyoxygenated cembrane-type diterpenes from the Hainan soft coral Lobophytum crassum as a promising source of anticancer agents with ErbB3 and ROR1 inhibitory potential.Acta Pharmacol Sin. 2025 Jan;46(1):196-207. doi: 10.1038/s41401-024-01347-z. Epub 2024 Jul 29. Acta Pharmacol Sin. 2025. PMID: 39075227
-
Six Undescribed Capnosane-Type Macrocyclic Diterpenoids from South China Sea Soft Coral Sarcophyton crassocaule: Structural Determination and Biological Evaluation.Mar Drugs. 2023 Dec 18;21(12):645. doi: 10.3390/md21120645. Mar Drugs. 2023. PMID: 38132966 Free PMC article.
-
Uncommon Capnosane Diterpenes with Neuroprotective Potential from South China Sea Soft Coral Sarcophyton boettgeri.Mar Drugs. 2022 Sep 25;20(10):602. doi: 10.3390/md20100602. Mar Drugs. 2022. PMID: 36286428 Free PMC article.
-
Cherbonolides M and N from a Formosan Soft Coral Sarcophyton cherbonnieri.Mar Drugs. 2021 May 1;19(5):260. doi: 10.3390/md19050260. Mar Drugs. 2021. PMID: 34062855 Free PMC article.
References
-
- Weinheimer A.J., Chang C.W.J., Matson J.A. Progress in the Chemistry of Organic Natural Products. Springer-Verlag, Wien GmbH; Wien, Austria: 1979. pp. 285–388.
-
- Polastro F., Golin S., Chianese G., Putra M.Y., Moriello A.S., de Petrocellis L., García V., Munoz E., Taglialatela-Scafeiti O., Appendino G. Neuroactive and anti-inflammatory frankincense cembranes: A structure-activity study. J. Nat. Prod. 2016;79:1762–1768. doi: 10.1021/acs.jnatprod.6b00141. - DOI - PubMed
-
- Vasamsetty L., Khan F.A., Mehta G. A model approach towards the polycyclic framework present in cembranoid natural products dissectolide A, plumarellide and mandapamate. Tetrahedron Lett. 2014;55:7068–7071. doi: 10.1016/j.tetlet.2014.10.141. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

