The MEK-ERK-MST1 Axis Potentiates the Activation of the Extrinsic Apoptotic Pathway during GDC-0941 Treatment in Jurkat T Cells
- PMID: 30795621
- PMCID: PMC6406719
- DOI: 10.3390/cells8020191
The MEK-ERK-MST1 Axis Potentiates the Activation of the Extrinsic Apoptotic Pathway during GDC-0941 Treatment in Jurkat T Cells
Abstract
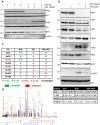
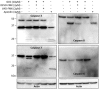
The discrete activation of individual caspases is essential during T-cell development, activation, and apoptosis. Humans carrying nonfunctional caspase-8 and caspase-8 conditional knockout mice exhibit several defects in the progression of naive CD4⁺ T cells to the effector stage. MST1, a key kinase of the Hippo signaling pathway, is often presented as a substrate of caspases, and its cleavage by caspases potentiates its activity. Several studies have focused on the involvement of MST1 in caspase activation and also reported several defects in the immune system function caused by MST1 deficiency. Here, we show the rapid activation of the MEK-ERK-MST1 axis together with the cleavage and activation of caspase-3, -6, -7, -8, and -9 after PI3K signaling blockade by the selective inhibitor GDC-0941 in Jurkat T cells. We determined the phosphorylation pattern of MST1 using a phosphoproteomic approach and identified two amino acid residues phosphorylated in an ERK-dependent manner after GDC-0941 treatment together with a novel phosphorylation site at S21 residue, which was extensively phosphorylated in an ERK-independent manner during PI3K signaling blockade. Using caspase inhibitors and the inhibition of MST1 expression using siRNA, we identified an exclusive role of the MEK-ERK-MST1 axis in the activation of initiator caspase-8, which in turn activates executive caspase-3/-7 that finally potentiate MST1 proteolytic cleavage. This mechanism forms a positive feed-back loop that amplifies the activation of MST1 together with apoptotic response in Jurkat T cells during PI3K inhibition. Altogether, we propose a novel MEK-ERK-MST1-CASP8-CASP3/7 apoptotic pathway in Jurkat T cells and believe that the regulation of this pathway can open novel possibilities in systemic and cancer therapies.
Keywords: AKT; ERK; Hippo/MST1; MEK; PI3K; apoptosis; caspase.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

