Effectiveness of a new multi-component smoking cessation service package for patients with hypertension and diabetes in northern Thailand: a randomized controlled trial (ESCAPE study)
- PMID: 30795811
- PMCID: PMC6387550
- DOI: 10.1186/s13011-019-0197-2
Effectiveness of a new multi-component smoking cessation service package for patients with hypertension and diabetes in northern Thailand: a randomized controlled trial (ESCAPE study)
Abstract
Background: Smoking cessation is an achievable behavioral change, which reduces the risks of cardiovascular diseases, cancers and tobacco-related diseases. There is a need for an effective smoking cessation service for low and middle income country settings where the smoking rate is generally very high whilst a cessation service is not usually accessible. This study devised a new smoking cessation service package and assessed its effectiveness in the primary health care setting of northern Thailand.
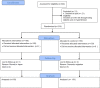
Methods: This randomized controlled trial was centered at Maetha district hospital, Lampang province, Thailand, and its network of mobile non-communicable disease clinics at seven primary care units. A total of 319 eligible patients who consented to participate in the study, were randomly allocated to an intervention arm (160) and a control arm (159), applying block randomization. The multi-component intervention service consisted of: (1) regular patient motivation by the same nurse over a 3-month period; (2) a monthly piCO+ Smokerlyzer test for 3 months; (3) continual assistance from a trained family member, using a smoking-cessation- diary; and (4) optional nicotine replacement chewing gum therapy. The control group received the routine service comprising of brief counseling and casual follow-up. Smoking cessation, confirmed by six months of abstinence and the piCo+ Smokerlyzer breath test, was compared between the two services after a year follow-up. The trial is registered as an international current control trial at the ISRCTN registry. ISRCTN89315117.
Results: The median age of the participants was 64 years, with females constituting 28.84%. Most of the participants smoke hand-rolled cigarettes (85%). The intervention arm participants achieved a significantly higher smoking cessation rate than the control arm 25.62% vs 11.32%, with an adjusted odd ratio of 2.95 and 95% confidence interval 1.55-5.61.
Conclusion: In relation to accessing smoking cessation services within the primary health care setting, participants who received the evidence-based intervention package were about three times more likely to succeed in giving up smoking than those who received the routine service. Utilizing community resources as major intervention components, the evidence from this trial may provide a useful and scalable smoking cessation intervention for low and middle income countries.
Trial registration: Current controlled trials ISRCTN89315117 . WHO international clinical trial identifier number: U1111-1145-6916; 3/2013.
Keywords: Coaching; Diary; Family; Hand-rolled cigarette; NRT; Smokerlyzer; Tobacco.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Juntendo University Ethical Committee, Japan, with approval number 2012194, and the Institutional Review Board at Boromarajonani College of Nursing, Lampang, Thailand, with approval number E2556/005. It was registered as an international current control trial at the ISRCTN registry. The ISRCTN registration number ISRCTN89315117 was assigned on 9th July 2013 [10]. Written consent was obtained from each of the participants after a thorough explanation was given about what would happen to them during the study, as well as their autonomous decision to give up smoking.
Consent for publication
Written informed consent for publication was obtained to publish the findings.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Aung MN, Yuasa M, Moolphate S, et al. Challenges for smoking cessation intervention as part of primary health Care Services in Developing Countries. J Smok Cessat. 2014;11(4):211–218. doi: 10.1017/jsc.2014.28. - DOI
-
- Picazo OF. Engendering Concerted National Efforts towards Improved Health Outcomes in the ASEAN: Status, Challenges, Targets, and Ways Forward. 2015.
-
- Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010;1:CD006936. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

