LncRNA EPB41L4A-AS1 regulates glycolysis and glutaminolysis by mediating nucleolar translocation of HDAC2
- PMID: 30796006
- PMCID: PMC6444057
- DOI: 10.1016/j.ebiom.2019.01.035
LncRNA EPB41L4A-AS1 regulates glycolysis and glutaminolysis by mediating nucleolar translocation of HDAC2
Erratum in
-
Corrigendum for "LncRNA EPB41L4A-AS1 regulates glycolysis and glutaminolysis by mediating nucleolar translocation of HDAC2" [eBioMedicine 41 (2019) 200-213].EBioMedicine. 2024 Apr;102:105084. doi: 10.1016/j.ebiom.2024.105084. Epub 2024 Mar 21. EBioMedicine. 2024. PMID: 38518654 Free PMC article. No abstract available.
Abstract
Background: LncRNAs have been found to be involved in various aspects of biological processes. In this study, we aimed to uncover the molecular mechanisms of lncRNA EPB41L4A-AS1 in regulating glycolysis and glutaminolysis in cancer cells.
Methods: The expression of EPB41L4A-AS1 in cancer patients was analyzed in TCGA and GEO datasets. The level of cellular metabolism was determined by extracellular flux analyzer. The relationship between p53 and EPB41L4A-AS1 was explored by qRT-PCR, luciferase assay and ChIP assay. The interactions between EPB41L4A-AS1 and HDAC2 or NPM1 were determined by RNA immunoprecipitation, RNA pull-down assay and RNA-FISH- immunofluorescence.
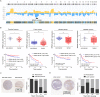
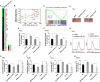
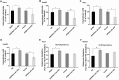
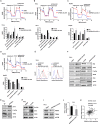
Findings: EPB41L4A-AS1 was a p53-regulated gene. Low expression and deletion of lncRNA EPB41L4A-AS1 were found in a variety of human cancers and associated with poor prognosis of cancer patients. Knock down EPB41L4A-AS1 expression triggered Warburg effect, demonstrated as increased aerobic glycolysis and glutaminolysis. EPB41L4A-AS1 interacted and colocalized with HDAC2 and NPM1 in nucleolus. Silencing EPB41L4A-AS1 reduced the interaction between HDAC2 and NPM1, released HDAC2 from nucleolus and increased its distribution in nucleoplasm, enhanced HDAC2 occupation on VHL and VDAC1 promoter regions, and finally accelerated glycolysis and glutaminolysis. Depletion of EPB41L4A-AS1 increased the sensitivity of tumor to glutaminase inhibitor in tumor therapy.
Interpretation: EPB41L4A-AS1 functions as a repressor of the Warburg effect and plays important roles in metabolic reprogramming of cancer.
Keywords: Cancer metabolism; EPB41L4A-AS1; Glutaminolysis; Glycolysis; HDAC2.
Copyright © 2019. Published by Elsevier B.V.
Figures




References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
-
- Schuurbiers O.C., Meijer T.W., Kaanders J.H., Looijen-Salamon M.G., de Geus-Oei L.F., van der Drift M.A. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. J Thorac Oncol. 2014;9(10):1485–1493. - PubMed
-
- Upadhyay M., Samal J., Kandpal M., Singh O.V., Vivekanandan P. The Warburg effect: insights from the past decade. Pharmacol Ther. 2013;137(3):318–330. - PubMed
-
- Koppenol W.H., Bounds P.L., Dang C.V. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

