Fratricide-resistant CD1a-specific CAR T cells for the treatment of cortical T-cell acute lymphoblastic leukemia
- PMID: 30796021
- PMCID: PMC6554538
- DOI: 10.1182/blood-2018-10-882944
Fratricide-resistant CD1a-specific CAR T cells for the treatment of cortical T-cell acute lymphoblastic leukemia
Abstract
Relapsed/refractory T-cell acute lymphoblastic leukemia (T-ALL) has a dismal outcome, and no effective targeted immunotherapies for T-ALL exist. The extension of chimeric antigen receptor (CAR) T cells (CARTs) to T-ALL remains challenging because the shared expression of target antigens between CARTs and T-ALL blasts leads to CART fratricide. CD1a is exclusively expressed in cortical T-ALL (coT-ALL), a major subset of T-ALL, and retained at relapse. This article reports that the expression of CD1a is mainly restricted to developing cortical thymocytes, and neither CD34+ progenitors nor T cells express CD1a during ontogeny, confining the risk of on-target/off-tumor toxicity. We thus developed and preclinically validated a CD1a-specific CAR with robust and specific cytotoxicity in vitro and antileukemic activity in vivo in xenograft models of coT-ALL, using both cell lines and coT-ALL patient-derived primary blasts. CD1a-CARTs are fratricide resistant, persist long term in vivo (retaining antileukemic activity in re-challenge experiments), and respond to viral antigens. Our data support the therapeutic and safe use of fratricide-resistant CD1a-CARTs for relapsed/refractory coT-ALL.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





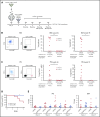

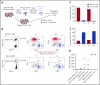
Comment in
-
Anti-CD1a CAR T cells to selectively target T-ALL.Blood. 2019 May 23;133(21):2246-2247. doi: 10.1182/blood-2019-03-900910. Blood. 2019. PMID: 31122936 No abstract available.
References
-
- Karrman K, Johansson B. Pediatric T-cell acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2017;56(2):89-116. - PubMed
-
- Weng AP, Ferrando AA, Lee W, et al. . Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269-271. - PubMed
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541-1552. - PubMed
-
- Litzow MR, Ferrando AA. How I treat T-cell acute lymphoblastic leukemia in adults. Blood. 2015;126(7):833-841. - PubMed
-
- Schneider NR, Carroll AJ, Shuster JJ, et al. . New recurring cytogenetic abnormalities and association of blast cell karyotypes with prognosis in childhood T-cell acute lymphoblastic leukemia: a pediatric oncology group report of 343 cases. Blood. 2000;96(7):2543-2549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

