Odd-skipped and Stripe act downstream of Notch to promote the morphogenesis of long appendicular tendons in Drosophila
- PMID: 30796048
- PMCID: PMC6451353
- DOI: 10.1242/bio.038760
Odd-skipped and Stripe act downstream of Notch to promote the morphogenesis of long appendicular tendons in Drosophila
Abstract
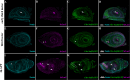
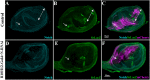
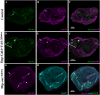
Multiple tissue interactions take place during the development of the limb musculoskeletal system. While appendicular myogenesis has been extensively studied, development of connective tissue associated with muscles has received less attention. In the developing Drosophila leg, tendon-like connective tissue arises from clusters of epithelial cells that invaginate into the leg cavity and then elongate to form internal tube-shape structures along which muscle precursors are distributed. Here we show that stripe-positive appendicular precursors of tendon-like connective tissue are set up among intersegmental leg joint cells expressing odd-skipped genes, and that Notch signaling is necessary and locally sufficient to trigger stripe expression. This study also finds that odd-skipped genes and stripe are both required downstream of Notch to promote morphogenesis of tube-shaped internal tendons of the leg.
Keywords: Drosophila leg; Muscle development; Notch; Odd-skipped; Tendon; Tubulogenesis.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
Transcriptomic and Genetic Analyses Identify the Krüppel-Like Factor Dar1 as a New Regulator of Tube-Shaped Long Tendon Development.Front Cell Dev Biol. 2021 Dec 16;9:747563. doi: 10.3389/fcell.2021.747563. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977007 Free PMC article.
-
The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation.Dev Biol. 2003 Nov 15;263(2):282-95. doi: 10.1016/j.ydbio.2003.07.011. Dev Biol. 2003. PMID: 14597202
-
Coordinated development of muscles and tendons of the Drosophila leg.Development. 2004 Dec;131(24):6041-51. doi: 10.1242/dev.01527. Epub 2004 Nov 10. Development. 2004. PMID: 15537687
-
Non-myogenic Contribution to Muscle Development and Homeostasis: The Role of Connective Tissues.Front Cell Dev Biol. 2017 Mar 23;5:22. doi: 10.3389/fcell.2017.00022. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28386539 Free PMC article. Review.
-
Drosophila adult muscle development and regeneration.Semin Cell Dev Biol. 2017 Dec;72:56-66. doi: 10.1016/j.semcdb.2017.11.017. Epub 2017 Nov 16. Semin Cell Dev Biol. 2017. PMID: 29146144 Review.
Cited by
-
Developmental origin of tendon diversity in Drosophila melanogaster.Front Physiol. 2023 Apr 18;14:1176148. doi: 10.3389/fphys.2023.1176148. eCollection 2023. Front Physiol. 2023. PMID: 37143929 Free PMC article. Review.
-
Transcriptomic and Genetic Analyses Identify the Krüppel-Like Factor Dar1 as a New Regulator of Tube-Shaped Long Tendon Development.Front Cell Dev Biol. 2021 Dec 16;9:747563. doi: 10.3389/fcell.2021.747563. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977007 Free PMC article.
-
Genetic Control of Muscle Diversification and Homeostasis: Insights from Drosophila.Cells. 2020 Jun 25;9(6):1543. doi: 10.3390/cells9061543. Cells. 2020. PMID: 32630420 Free PMC article. Review.
References
-
- Abu-Shaar M. and Mann R. S. (1998). Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development 125, 3821-3830. - PubMed
-
- Becker S., Pasca G., Strumpf D., Min L. and Volk T. (1997). Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development 124, 2615-2622. - PubMed
-
- Bishop S. A., Klein T., Arias A. M. and Couso J. P. (1999). Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development 126, 2993-3003. - PubMed
-
- Brand A. H. and Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. - PubMed
-
- de Celis J. F., Tyler D. M., de Celis J. and Bray S. J. (1998). Notch signalling mediates segmentation of the Drosophila leg. Development 125, 4617-4626. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

