Coculturing Bacteria Leads to Reduced Phenotypic Heterogeneities
- PMID: 30796063
- PMCID: PMC6450022
- DOI: 10.1128/AEM.02814-18
Coculturing Bacteria Leads to Reduced Phenotypic Heterogeneities
Abstract
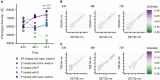
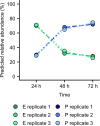
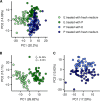
Isogenic bacterial populations are known to exhibit phenotypic heterogeneity at the single-cell level. Because of difficulties in assessing the phenotypic heterogeneity of a single taxon in a mixed community, the importance of this deeper level of organization remains relatively unknown for natural communities. In this study, we have used membrane-based microcosms that allow the probing of the phenotypic heterogeneity of a single taxon while interacting with a synthetic or natural community. Individual taxa were studied under axenic conditions, as members of a coculture with physical separation, and as a mixed culture. Phenotypic heterogeneity was assessed through both flow cytometry and Raman spectroscopy. Using this setup, we investigated the effect of microbial interactions on the individual phenotypic heterogeneities of two interacting drinking water isolates. Through flow cytometry we have demonstrated that interactions between these bacteria lead to a reduction of their individual phenotypic diversities and that this adjustment is conditional on the bacterial taxon. Single-cell Raman spectroscopy confirmed a taxon-dependent phenotypic shift due to the interaction. In conclusion, our data suggest that bacterial interactions may be a general driver of phenotypic heterogeneity in mixed microbial populations.IMPORTANCE Laboratory studies have shown the impact of phenotypic heterogeneity on the survival and functionality of isogenic populations. Because phenotypic heterogeneity plays an important role in pathogenicity and virulence, antibiotic resistance, biotechnological applications, and ecosystem properties, it is crucial to understand its influencing factors. An unanswered question is whether bacteria in mixed communities influence the phenotypic heterogeneity of their community partners. We found that coculturing bacteria leads to a reduction in their individual phenotypic heterogeneities, which led us to the hypothesis that the individual phenotypic diversity of a taxon is dependent on the community composition.
Keywords: Raman spectroscopy; axenic culture; coculture; flow cytometry; microbial interactions; phenotypic heterogeneity; single cell; synthetic ecosystems.
Copyright © 2019 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

