Prospective study of Burkitt lymphoma treatment in adolescents and adults in Malawi
- PMID: 30796065
- PMCID: PMC6391663
- DOI: 10.1182/bloodadvances.2018029199
Prospective study of Burkitt lymphoma treatment in adolescents and adults in Malawi
Abstract
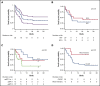
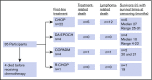
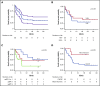
Burkitt lymphoma (BL) is common in sub-Saharan Africa (SSA). In high-income countries, BL is highly curable with chemotherapy. However, there are few prospective studies from SSA describing nonpediatric BL and no regional standard of care. Thirty-five participants age 15 years or older with newly diagnosed BL were enrolled in Malawi from 2013 to 2018. Chemotherapy was administered according to institutional guidelines, with concurrent antiretroviral therapy if HIV infected. Median age was 21 years (range, 15-61) and 15 participants (43%) were HIV infected. Twenty-seven participants (77%) had stage III to IV disease, and 19 (54%) had Eastern Cooperative Oncology Group performance status >1. Among HIV-infected participants, median CD4 count was 130 (range, 29-605) and 10 (67%) had suppressed HIV viral load. Four participants (11%) died before receiving chemotherapy. First-line chemotherapy consisted of: cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (n = 22 [71%]); infusional etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (n = 4 [13%]); high-dose methotrexate-based chemotherapy (n = 4 [13%]); and rituximab plus CHOP (n = 1 [3%]). Among 28 evaluable participants, 14 (50%) achieved a complete response. Median overall survival (OS) was 7 months; 1-year OS was 40% (95% confidence interval [CI], 24%-56%). Sixteen (73%) of 22 deaths were a result of disease progression. Compared with CHOP, more intensive chemotherapy was associated with decreased mortality (hazard ratio, 0.24; 95% CI, 0.05-1.02; P = .05). This is among the best characterized prospective cohorts of nonpediatric BL in SSA. Most deaths resulted from progressive BL. Patients who received more intensive therapy seemed to have better outcomes. Defining optimal approaches is an urgent priority in SSA.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Sinfield RL, Molyneux EM, Banda K, et al. Spectrum and presentation of pediatric malignancies in the HIV era: experience from Blantyre, Malawi, 1998-2003. Pediatr Blood Cancer. 2007;48(5):515-520. - PubMed
-
- Engels EA, Pfeiffer RM, Goedert JJ, et al. ; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20(12):1645-1654. - PubMed
-
- Patte C, Auperin A, Michon J, et al. ; Société Française d’Oncologie Pédiatrique. The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97(11):3370-3379. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

