Antidepressants are modifiers of lipid bilayer properties
- PMID: 30796095
- PMCID: PMC6400527
- DOI: 10.1085/jgp.201812263
Antidepressants are modifiers of lipid bilayer properties
Abstract
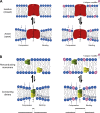
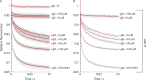
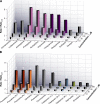
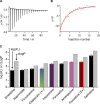
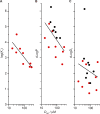
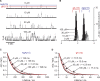
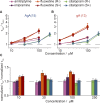
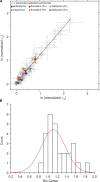
The two major classes of antidepressants, tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs), inhibit neurotransmitter reuptake at synapses. They also have off-target effects on proteins other than neurotransmitter transporters, which may contribute to both desired changes in brain function and the development of side effects. Many proteins modulated by antidepressants are bilayer spanning and coupled to the bilayer through hydrophobic interactions such that the conformational changes underlying their function will perturb the surrounding lipid bilayer, with an energetic cost (ΔG def) that varies with changes in bilayer properties. Here, we test whether changes in ΔG def caused by amphiphilic antidepressants partitioning into the bilayer are sufficient to alter membrane protein function. Using gramicidin A (gA) channels to probe whether TCAs and SSRIs alter the bilayer contribution to the free energy difference for the gramicidin monomer⇔dimer equilibrium (representing a well-defined conformational transition), we find that antidepressants alter gA channel activity with varying potency and no stereospecificity but with different effects on bilayer elasticity and intrinsic curvature. Measuring the antidepressant partition coefficients using isothermal titration calorimetry (ITC) or cLogP shows that the bilayer-modifying potency is predicted quite well by the ITC-determined partition coefficients, and channel activity is doubled at an antidepressant/lipid mole ratio of 0.02-0.07. These results suggest a mechanism by which antidepressants could alter the function of diverse membrane proteins by partitioning into cell membranes and thereby altering the bilayer contribution to the energetics of membrane protein conformational changes.
© 2019 Kapoor et al.
Figures
References
-
- ACD/Percepta PhysChem Suite 2012. ACD/Percepta PhysChem Suite. Available at: https://www.acdlabs.com/products/percepta/ (accessed December 19, 2018).
-
- Andersen O.S., Sawyer D.B., and Koeppe R.E. II. 1992. Modulation of channel function by the host bilayer. In Biomembrane Structure and Function: The State of the Art. Gaber B.P., and Easwaran K.R.K., editors. Adenine Press, Schenectady, NY: 227–244.
-
- Avdeef A. 2001. Physicochemical profiling (solubility, permeability and charge state). Curr. Top. Med. Chem. 1:277–351. - PubMed
-
- Aveyard R., and Haydon D.A.. 1973. An Introduction to the Principles of Surface Chemistry. Cambridge University Press, London.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

