Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study
- PMID: 30796151
- PMCID: PMC6656514
- DOI: 10.1634/theoncologist.2018-0618
Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study
Abstract
Background: Patients with a history of autoimmune diseases (AIDs) have not usually been included in clinical trials with immune checkpoint inhibitors.
Materials and methods: Consecutive patients with advanced cancer, treated with anti-programmed death-1 (PD-1) agents, were evaluated according to the presence of pre-existing AIDs. The incidence of immune-related adverse events (irAEs) and clinical outcomes were compared among subgroups.
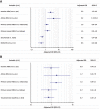
Results: A total of 751 patients were enrolled; median age was 69 years. Primary tumors were as follows: non-small cell lung cancer, 492 (65.5%); melanoma, 159 (21.2%); kidney cancer, 94 (12.5%); and others, 6 (0.8%). Male/female ratio was 499/252. Eighty-five patients (11.3%) had pre-existing AIDs, further differentiated in clinically active (17.6%) and inactive (82.4%). Among patients with pre-existing AIDs, incidence of irAEs of any grade was significantly higher when compared with patients without AIDs (65.9% vs. 39.9%). At multivariate analysis, both inactive (p = .0005) and active pre-existing AIDs (p = .0162), female sex (p = .0004), and Eastern Cooperative Oncology Group Performance Status <2 (p = .0030) were significantly related to a higher incidence of irAEs of any grade. No significant differences were observed regarding grade 3/4 irAEs and objective response rate among subgroups. Pre-existing AIDs were not significantly related with progression-free survival and overall survival.
Conclusion: This study quantifies the increased risk of developing irAEs in patients with pre-existing AIDs who had to be treated with anti-PD-1 immunotherapy. Nevertheless, the incidence of grade 3/4 irAEs is not significantly higher when compared with control population. The finding of a greater incidence of irAEs among female patients ranks among the "hot topics" in gender-related differences in immuno-oncology.
Implications for practice: Patients with a history of autoimmune diseases (AIDs) have not usually been included in clinical trials with immune checkpoint inhibitors but are frequent in clinical practice. This study quantifies the increased risk of developing immune-related adverse events (irAEs) in patients with pre-existing AIDs who had to be treated with anti-programmed death-1 immunotherapy. Nevertheless, their toxicities are mild and the incidence of grade 3/4 irAEs is not significantly higher compared with those of controls. These results will help clinicians in everyday practice, improving their ability to offer a proper counselling to patients, in order to offer an immunotherapy treatment even to patients with pre-existing autoimmune disease.
Keywords: Anti‐programmed death‐1; Autoimmune disease; Immune checkpoint inhibitors; Immunotherapy; Performance status; Sex.
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 2016;27:559–574. - PubMed
-
- Johnson DB, Sullivan RJ, Ott PA et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–240. - PubMed
-
- Menzies AM, Johnson DB, Ramanujam S et al. Anti‐PD‐1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–376. - PubMed
-
- Abdel‐Wahab N, Shah M, Lopez‐Olivo MA et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann Intern Med 2018;168:121–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

