Parkinson's disease-associated mutations in the GTPase domain of LRRK2 impair its nucleotide-dependent conformational dynamics
- PMID: 30796162
- PMCID: PMC6463707
- DOI: 10.1074/jbc.RA119.007631
Parkinson's disease-associated mutations in the GTPase domain of LRRK2 impair its nucleotide-dependent conformational dynamics
Abstract
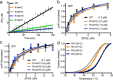
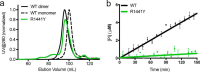
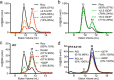
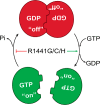
Mutation in leucine-rich repeat kinase 2 (LRRK2) is a common cause of familial Parkinson's disease (PD). Recently, we showed that a disease-associated mutation R1441H rendered the GTPase domain of LRRK2 catalytically less active and thereby trapping it in a more persistently "on" conformation. However, the mechanism involved and characteristics of this on conformation remained unknown. Here, we report that the Ras of complex protein (ROC) domain of LRRK2 exists in a dynamic dimer-monomer equilibrium that is oppositely driven by GDP and GTP binding. We also observed that the PD-associated mutations at residue 1441 impair this dynamic and shift the conformation of ROC to a GTP-bound-like monomeric conformation. Moreover, we show that residue Arg-1441 is critical for regulating the conformational dynamics of ROC. In summary, our results reveal that the PD-associated substitutions at Arg-1441 of LRRK2 alter monomer-dimer dynamics and thereby trap its GTPase domain in an activated state.
Keywords: GTPase; Parkinson disease; Ras of complex proteins (ROC); conformational change; conformational dynamics; disease mutation; enzyme activation; kinase; leucine-rich repeat kinase 2 (LRRK2); molecular dynamics.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Markov State Models and Molecular Dynamics Simulations Provide Understanding of the Nucleotide-Dependent Dimerization-Based Activation of LRRK2 ROC Domain.Molecules. 2021 Sep 17;26(18):5647. doi: 10.3390/molecules26185647. Molecules. 2021. PMID: 34577121 Free PMC article.
-
The Parkinson's disease-associated mutation N1437H impairs conformational dynamics in the G domain of LRRK2.FASEB J. 2019 Apr;33(4):4814-4823. doi: 10.1096/fj.201802031R. Epub 2018 Dec 28. FASEB J. 2019. PMID: 30592623 Free PMC article.
-
The Roc-COR tandem domain of leucine-rich repeat kinase 2 forms dimers and exhibits conventional Ras-like GTPase properties.J Neurochem. 2018 Nov;147(3):409-428. doi: 10.1111/jnc.14566. J Neurochem. 2018. PMID: 30091236
-
Understanding the GTPase Activity of LRRK2: Regulation, Function, and Neurotoxicity.Adv Neurobiol. 2017;14:71-88. doi: 10.1007/978-3-319-49969-7_4. Adv Neurobiol. 2017. PMID: 28353279 Free PMC article. Review.
-
The unconventional G-protein cycle of LRRK2 and Roco proteins.Biochem Soc Trans. 2016 Dec 15;44(6):1611-1616. doi: 10.1042/BST20160224. Biochem Soc Trans. 2016. PMID: 27913669 Review.
Cited by
-
Oligomerization of Lrrk controls actin severing and α-synuclein neurotoxicity in vivo.Mol Neurodegener. 2021 May 24;16(1):33. doi: 10.1186/s13024-021-00454-3. Mol Neurodegener. 2021. PMID: 34030727 Free PMC article.
-
Overview of the Impact of Pathogenic LRRK2 Mutations in Parkinson's Disease.Biomolecules. 2023 May 16;13(5):845. doi: 10.3390/biom13050845. Biomolecules. 2023. PMID: 37238714 Free PMC article. Review.
-
Allosteric inhibition of LRRK2, where are we now.Biochem Soc Trans. 2020 Oct 30;48(5):2185-2194. doi: 10.1042/BST20200424. Biochem Soc Trans. 2020. PMID: 33079169 Free PMC article. Review.
-
Binding of the Human 14-3-3 Isoforms to Distinct Sites in the Leucine-Rich Repeat Kinase 2.Front Neurosci. 2020 Apr 7;14:302. doi: 10.3389/fnins.2020.00302. eCollection 2020. Front Neurosci. 2020. PMID: 32317922 Free PMC article.
-
Markov State Models and Molecular Dynamics Simulations Provide Understanding of the Nucleotide-Dependent Dimerization-Based Activation of LRRK2 ROC Domain.Molecules. 2021 Sep 17;26(18):5647. doi: 10.3390/molecules26185647. Molecules. 2021. PMID: 34577121 Free PMC article.
References
-
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R. J., Calne D. B., Stoessl A. J., Pfeiffer R. F., Patenge N., Carbajal I. C., Vieregge P., et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 10.1016/j.neuron.2004.11.005 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

