Photoexcitation-controlled self-recoverable molecular aggregation for flicker phosphorescence
- PMID: 30796185
- PMCID: PMC6421427
- DOI: 10.1073/pnas.1821991116
Photoexcitation-controlled self-recoverable molecular aggregation for flicker phosphorescence
Abstract
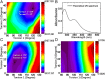
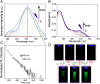
Chemical systems with external control capability and self-recoverability are promising since they can avoid additional chemical or energy imposition during the working process. However, it remains challenging to employ such a nonequilibrium method for the engineering of optoelectronic function and for visualization. Here, we report a functional molecule that can undergo intense conformational regulation upon photoexcitation. It enables a dynamical change in hydrophobicity and a follow-up molecular aggregation in aqueous media, accordingly leading to an aggregation-induced phosphorescence (AIP) behavior. This successive performance is self-recoverable, allowing a rapid (second-scale cycle) and long-standing (>103 cycles) flicker ability under rhythmical control of the AIP. Compared with traditional bidirectional manipulations, such monodirectional photocontrol with spontaneous reset profoundly enhances the operability while mostly avoiding possible side reactions and fatigue accumulation. Furthermore, this material can serve as a type of luminescent probe for dynamically strengthening visualization in bioimaging.
Keywords: aggregation-induced phosphorescence; conformational regulation; luminescent probe; photoexcitation; self-recoverable.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Coskun A, Banaszak M, Astumian RD, Stoddart JF, Grzybowski BA. Great expectations: Can artificial molecular machines deliver on their promise? Chem Soc Rev. 2012;41:19–30. - PubMed
-
- Sauvage JP. From chemical topology to molecular machines (Nobel lecture) Angew Chem Int Ed Engl. 2017;56:11080–11093. - PubMed
-
- De Bo G, et al. An artificial molecular machine that builds an asymmetric catalyst. Nat Nanotechnol. 2018;13:381–385. - PubMed
-
- Feringa BL. The art of building small: From molecular switches to motors (Nobel lecture) Angew Chem Int Ed Engl. 2017;56:11060–11078. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

