Elementary response triggered by transducin in retinal rods
- PMID: 30796193
- PMCID: PMC6421417
- DOI: 10.1073/pnas.1817781116
Elementary response triggered by transducin in retinal rods
Abstract
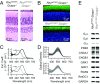
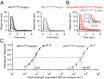
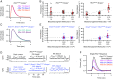
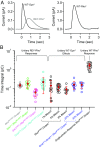
G protein-coupled receptor (GPCR) signaling is crucial for many physiological processes. A signature of such pathways is high amplification, a concept originating from retinal rod phototransduction, whereby one photoactivated rhodopsin molecule (Rho*) was long reported to activate several hundred transducins (GT*s), each then activating a cGMP-phosphodiesterase catalytic subunit (GT*·PDE*). This high gain at the Rho*-to-GT* step has been challenged more recently, but estimates remain dispersed and rely on some nonintact rod measurements. With two independent approaches, one with an extremely inefficient mutant rhodopsin and the other with WT bleached rhodopsin, which has exceedingly weak constitutive activity in darkness, we obtained an estimate for the electrical effect from a single GT*·PDE* molecular complex in intact mouse rods. Comparing the single-GT*·PDE* effect to the WT single-photon response, both in Gcaps-/- background, gives an effective gain of only ∼12-14 GT*·PDE*s produced per Rho*. Our findings have finally dispelled the entrenched concept of very high gain at the receptor-to-G protein/effector step in GPCR systems.
Keywords: G protein-coupled receptor; apo-opsin; rhodopsin; rod phototransduction; signal amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Phototransduction gain at the G-protein, transducin, and effector protein, phosphodiesterase-6, stages in retinal rods.Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):8653-8654. doi: 10.1073/pnas.1904017116. Proc Natl Acad Sci U S A. 2019. PMID: 31040258 Free PMC article. No abstract available.
References
-
- Vuong TM, Chabre M, Stryer L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature. 1984;311:659–661. - PubMed
-
- Leskov IB, et al. The gain of rod phototransduction: Reconciliation of biochemical and electrophysiological measurements. Neuron. 2000;27:525–537. - PubMed
-
- Alberts B, et al. Molecular Biology of the Cell. 6th Ed. Garland Science; New York: 2015. Second messengers and enzymatic cascades amplify signals; p. 869.
-
- Kandel ER, Schwartz JH, Jessel TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. 5th Ed. McGraw-Hill Education; New York: 2012. Low-level visual processing: The retina; p. 583.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

