Prototype of running clinical trials in an untrustworthy environment using blockchain
- PMID: 30796226
- PMCID: PMC6384889
- DOI: 10.1038/s41467-019-08874-y
Prototype of running clinical trials in an untrustworthy environment using blockchain
Abstract
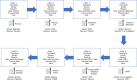
Monitoring and ensuring the integrity of data within the clinical trial process is currently not always feasible with the current research system. We propose a blockchain-based system to make data collected in the clinical trial process immutable, traceable, and potentially more trustworthy. We use raw data from a real completed clinical trial, simulate the trial onto a proof of concept web portal service, and test its resilience to data tampering. We also assess its prospects to provide a traceable and useful audit trail of trial data for regulators, and a flexible service for all members within the clinical trials network. We also improve the way adverse events are currently reported. In conclusion, we advocate that this service could offer an improvement in clinical trial data management, and could bolster trust in the clinical research process and the ease at which regulators can oversee trials.
Conflict of interest statement
The authors declare no competing interests.
Figures