Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation
- PMID: 30796228
- PMCID: PMC6385291
- DOI: 10.1038/s41467-019-08796-9
Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation
Abstract
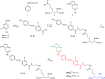
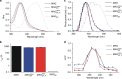
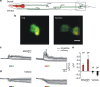
Manipulation of neuronal activity using two-photon excitation of azobenzene photoswitches with near-infrared light has been recently demonstrated, but their practical use in neuronal tissue to photostimulate individual neurons with three-dimensional precision has been hampered by firstly, the low efficacy and reliability of NIR-induced azobenzene photoisomerization compared to one-photon excitation, and secondly, the short cis state lifetime of the two-photon responsive azo switches. Here we report the rational design based on theoretical calculations and the synthesis of azobenzene photoswitches endowed with both high two-photon absorption cross section and slow thermal back-isomerization. These compounds provide optimized and sustained two-photon neuronal stimulation both in light-scattering brain tissue and in Caenorhabditis elegans nematodes, displaying photoresponse intensities that are comparable to those achieved under one-photon excitation. This finding opens the way to use both genetically targeted and pharmacologically selective azobenzene photoswitches to dissect intact neuronal circuits in three dimensions.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

