Heterosubtypic cross-protection correlates with cross-reactive interferon-gamma-secreting lymphocytes in the ferret model of influenza
- PMID: 30796267
- PMCID: PMC6384896
- DOI: 10.1038/s41598-019-38885-0
Heterosubtypic cross-protection correlates with cross-reactive interferon-gamma-secreting lymphocytes in the ferret model of influenza
Abstract
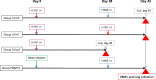
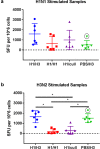
An effective universal vaccine for influenza will likely need to induce virus-specific T-cells, which are the major mediator of heterosubtypic cross-protection between different subtypes of influenza A virus. In this study we characterise the cell-mediated immune response in ferrets during heterosubtypic protection induced by low-dose H1N1 virus infection against an H3N2 virus challenge, given 4 weeks later. Although the ferrets were not protected against the infection by H3N2 virus, the duration of virus shedding was shortened, and clinical disease was markedly reduced. No cross-reactive neutralizing antibodies were detected, but cross-reactive interferon-gamma-secreting T cells were detected in the circulation prior to H3N2 challenge. These T-cells peaked at 11 days post-H1N1 infection, and were strongly induced in blood and in lung following H3N2 infection. The rapid induction of interferon-gamma-secreting cells in ferrets previously infected with H1N1 virus, but not in naïve ferrets, suggests induction of memory T-cells. These results are in accord with the observations that pre-existing cross-reactive T-cells correlate with protection in humans and have implications for outbreak modelling and universal vaccine design.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- World Health Organization Influenza (seasonal) fact sheet. http://www.who.int/mediacentre/factsheets/fs211/en/ Accessed 14-feb-18 (2018).
-
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scandinavian journal of immunology. 2004;59:1–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

