Mutations in the palm domain disrupt modulation of acid-sensing ion channel 1a currents by neuropeptides
- PMID: 30796301
- PMCID: PMC6385203
- DOI: 10.1038/s41598-018-37426-5
Mutations in the palm domain disrupt modulation of acid-sensing ion channel 1a currents by neuropeptides
Abstract
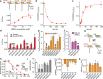
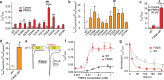
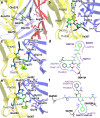
Modulation by neuropeptides enhances several functions of acid-sensing ion channels (ASICs), such as pain sensation and acid-induced neuronal injury. The acid-induced opening of ASICs is transient, because of a rapid desensitization. Neuropeptides containing an Arg-Phe-amide motif affect ASIC desensitization and allow continuous activity of ASICs. In spite of the importance of the sustained ASIC activity during prolonged acidification, the molecular mechanisms of ASIC modulation by neuropeptides is only poorly understood. To identify the FRRFa (Phe-Arg-Arg-Phe-amide) binding site on ASIC1a, we carried out an in silico docking analysis and verified functionally the docking predictions. The docking experiments indicated three possible binding pockets, located (1) in the acidic pocket between the thumb, finger, β-ball and palm domains, (2) in a pocket at the bottom of the thumb domain, and (3) in the central vestibule along with the connected side cavities. Functional measurements of mutant ASIC1a confirmed the importance of residues of the lower palm, which encloses the central vestibule and its side cavities, for the FRRFa effects. The combined docking and functional experiments strongly suggest that FRRFa binds to the central vestibule and its side cavities to change ASIC desensitization.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

