STING palmitoylation as a therapeutic target
- PMID: 30796349
- PMCID: PMC6460494
- DOI: 10.1038/s41423-019-0205-5
STING palmitoylation as a therapeutic target
Abstract
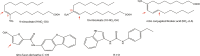
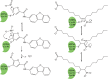
Gain-of-function mutations in the STING-encoding gene TMEM173 are central to the pathology of the autoinflammatory disorder STING-associated vasculopathy with onset in infancy (SAVI). Furthermore, excessive activity of the STING signaling pathway is associated with autoinflammatory diseases, including systemic lupus erythematosus and Aicardi-Goutières syndrome (AGS). Two independent studies recently identified pharmacological inhibitors of STING. Strikingly, both types of compounds are reactive nitro-containing electrophiles that target STING palmitoylation, a posttranslational modification necessary for STING signaling. As a consequence, the activation of downstream signaling molecules and the induction of type I interferons were inhibited. The compounds were effective at ameliorating inflammation in a mouse model of AGS and in blocking the production of type I interferons in primary fibroblasts from SAVI patients. This mini-review focuses on the roles of palmitoylation in STING activation and signaling and as a pharmaceutical target for drug development.
Keywords: Inflammation; Interferonopathies; Palmitoylation; SAVI; STING.
Conflict of interest statement
F.J.S. has a financial interest in Complexa Inc. The remaining authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

