Engineering for Success: Approaches to Improve Chimeric Antigen Receptor T Cell Therapy for Solid Tumors
- PMID: 30796733
- PMCID: PMC6613829
- DOI: 10.1007/s40265-019-01071-7
Engineering for Success: Approaches to Improve Chimeric Antigen Receptor T Cell Therapy for Solid Tumors
Abstract
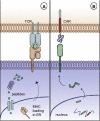
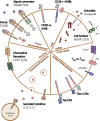
While impressive clinical responses have been observed using chimeric antigen receptor (CAR) T cells targeting CD19+ hematologic malignancies, limited clinical benefit has been observed using CAR T cells for a variety of solid tumors. Results of clinical studies have highlighted several obstacles which CAR T cells face in the context of solid tumors, including insufficient homing to tumor sites, lack of expansion and persistence, encountering a highly immunosuppressive tumor microenvironment, and heterogeneous antigen expression. In this review, we review clinical outcomes and discuss strategies to improve the antitumor activity of CAR T cells for solid tumors.
Conflict of interest statement
Melinda Mata has no conflict of interest; she is currently an employee of Immatics US, Inc. Stephen Gottschalk has patents and patent applications in the field of T cell therapy and gene therapy for cancer, is a consultant of ViraCyte, LLC, a member of the data safety monitoring board of Immatics US, Inc., and received research funding from Tessa Therapeutics LTD.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

