Eukaryotic initiation factor 4A (eIF4A) during viral infections
- PMID: 30796742
- PMCID: PMC7088766
- DOI: 10.1007/s11262-019-01641-7
Eukaryotic initiation factor 4A (eIF4A) during viral infections
Abstract
The helicase eIF4A is part of the cellular eIF4F translation initiation complex. The main functions of eIF4A are to remove secondary complex structures within the 5'-untranslated region and to displace proteins attached to mRNA. As intracellular parasites, viruses regulate the processes involved in protein synthesis, and different mechanisms related to controlling translation factors, such as eIF4A, have been found. The inhibitors of this factor are currently known; these substances could be used in the near future as part of antiviral pharmacological therapies in instances of replication cycles in which eIF4A is required. In this review, the particularities of how some viruses make use of this initiation factor to synthesize their proteins are discussed.
Keywords: Initiation; Translation; Virus; eIF4A.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
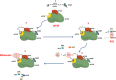
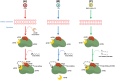
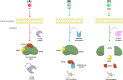
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

