Dlx1/2 are Central and Essential Components in the Transcriptional Code for Generating Olfactory Bulb Interneurons
- PMID: 30796806
- PMCID: PMC6917526
- DOI: 10.1093/cercor/bhz018
Dlx1/2 are Central and Essential Components in the Transcriptional Code for Generating Olfactory Bulb Interneurons
Abstract
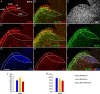
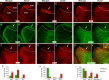
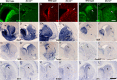
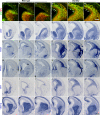
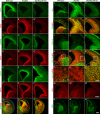
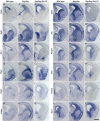
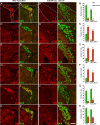
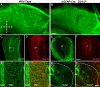
Generation of olfactory bulb (OB) interneurons requires neural stem/progenitor cell specification, proliferation, differentiation, and young interneuron migration and maturation. Here, we show that the homeobox transcription factors Dlx1/2 are central and essential components in the transcriptional code for generating OB interneurons. In Dlx1/2 constitutive null mutants, the differentiation of GSX2+ and ASCL1+ neural stem/progenitor cells in the dorsal lateral ganglionic eminence is blocked, resulting in a failure of OB interneuron generation. In Dlx1/2 conditional mutants (hGFAP-Cre; Dlx1/2F/- mice), GSX2+ and ASCL1+ neural stem/progenitor cells in the postnatal subventricular zone also fail to differentiate into OB interneurons. In contrast, overexpression of Dlx1&2 in embryonic mouse cortex led to ectopic production of OB-like interneurons that expressed Gad1, Sp8, Sp9, Arx, Pbx3, Etv1, Tshz1, and Prokr2. Pax6 mutants generate cortical ectopia with OB-like interneurons, but do not do so in compound Pax6; Dlx1/2 mutants. We propose that DLX1/2 promote OB interneuron development mainly through activating the expression of Sp8/9, which further promote Tshz1 and Prokr2 expression. Based on this study, in combination with earlier ones, we propose a transcriptional network for the process of OB interneuron development.
Keywords: Ascl1, Pax6; Dlx1; Dlx2; Gsx2; Sp8; Sp9; interneuron; olfactory bulb.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Allen ZJ 2nd, Waclaw RR, Colbert MC, Campbell K. 2007. Molecular identity of olfactory bulb interneurons: transcriptional codes of periglomerular neuron subtypes. J Mol Histol. 38:517–525. - PubMed
-
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. 2008. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 73:357–365. - PubMed
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. 1997. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 19:27–37. - PubMed
-
- Bartolini G, Ciceri G, Marin O. 2013. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 79:849–864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

