Cardiac innervation in acute myocardial ischaemia/reperfusion injury and cardioprotection
- PMID: 30796814
- PMCID: PMC6529901
- DOI: 10.1093/cvr/cvz053
Cardiac innervation in acute myocardial ischaemia/reperfusion injury and cardioprotection
Abstract
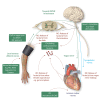
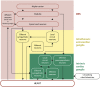
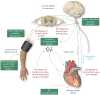
Acute myocardial infarction (AMI) and the heart failure (HF) that often complicates this condition, are among the leading causes of death and disability worldwide. To reduce myocardial infarct (MI) size and prevent heart failure, novel therapies are required to protect the heart against the detrimental effects of acute ischaemia/reperfusion injury (IRI). In this regard, targeting cardiac innervation may provide a novel therapeutic strategy for cardioprotection. A number of cardiac neural pathways mediate the beneficial effects of cardioprotective strategies such as ischaemic preconditioning and remote ischaemic conditioning, and nerve stimulation may therefore provide a novel therapeutic strategy for cardioprotection. In this article, we provide an overview of cardiac innervation and its impact on acute myocardial IRI, the role of extrinsic and intrinsic cardiac neural pathways in cardioprotection, and highlight peripheral and central nerve stimulation as a cardioprotective strategy with therapeutic potential for reducing MI size and preventing HF following AMI. This article is part of a Cardiovascular Research Spotlight Issue entitled 'Cardioprotection Beyond the Cardiomyocyte', and emerged as part of the discussions of the European Union (EU)-CARDIOPROTECTION Cooperation in Science and Technology (COST) Action, CA16225.
Keywords: Cardioprotection; Ischaemia/reperfusion injury; Myocardial infarction; Nervous system.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 2008;93:165–176. - PubMed
-
- Wake E, Brack K.. Characterization of the intrinsic cardiac nervous system. Auton Neurosci 2016;199:3–16. - PubMed
-
- Gatti PJ, Johnson TA, Phan P, Jordan IK III, Coleman W, Massari VJ.. The physiological and anatomical demonstration of functionally selective parasympathetic ganglia located in discrete fat pads on the feline myocardium. J Auton Nerv Syst 1995;51:255–259. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

