Structural characterization of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using EPR spectroscopy
- PMID: 30796886
- PMCID: PMC7212766
- DOI: 10.1016/j.chemphyslip.2019.02.003
Structural characterization of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using EPR spectroscopy
Abstract
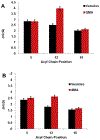
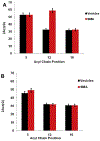
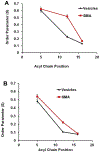
Spectroscopic studies of membrane proteins (MPs) are challenging due to difficulties in preparing homogenous and functional lipid membrane mimetic systems into which membrane proteins can properly fold and function. It has recently been shown that styrene-maleic acid (SMA) copolymers act as a macromolecular surfactant and therefore facilitate the formation of disk-shaped lipid bilayer nanoparticles (styrene-maleic acid copolymer-lipid nanoparticles (SMALPs)) that retain structural characteristics of native lipid membranes. We have previously reported controlled synthesis of SMA block copolymers using reversible addition-fragmentation chain transfer (RAFT) polymerization, and that alteration of the weight ratio of styrene to maleic acid affects nanoparticle size. RAFT-synthesis offers superior control over SMA polymer architecture compared to conventional radical polymerization techniques used for commercially available SMA. However, the interactions between the lipid bilayer and the solubilized RAFT-synthesized SMA polymer are currently not fully understood. In this study, EPR spectroscopy was used to detect the perturbation on the acyl chain upon introduction of the RAFT-synthesized SMA polymer by attaching PC-based nitroxide spin labels to the 5th, 12th, and 16th positions along the acyl chain of the lipid bilayer. EPR spectra showed high rigidity at the 12th position compared to the other two regions, displaying similar qualities to commercially available polymers synthesized via conventional methods. In addition, central EPR linewidths and correlation time data were obtained that are consistent with previous findings.
Keywords: CW-EPR; Electron paramagnetic resonance; Lipid bilayer; POPC vesicles; SMALPs; Styrene-maleic acid copolymers.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Competing financial interest
The authors declare no competing financial interest.
Figures


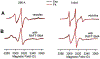
Similar articles
-
Tuning the size of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using RAFT polymerization for biophysical studies.Biochim Biophys Acta. 2016 Nov;1858(11):2931-2939. doi: 10.1016/j.bbamem.2016.08.004. Epub 2016 Aug 15. Biochim Biophys Acta. 2016. PMID: 27539205
-
Characterizing the structure of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using RAFT polymerization for membrane protein spectroscopic studies.Chem Phys Lipids. 2019 Jan;218:65-72. doi: 10.1016/j.chemphyslip.2018.12.002. Epub 2018 Dec 4. Chem Phys Lipids. 2019. PMID: 30528635 Free PMC article.
-
Influence of lipid saturation on the structural properties of styrene maleic acid lipid nanoparticles (SMALPs).Biochim Biophys Acta Biomembr. 2025 Jun;1867(5-6):184424. doi: 10.1016/j.bbamem.2025.184424. Epub 2025 May 22. Biochim Biophys Acta Biomembr. 2025. PMID: 40412742
-
Membrane proteins: is the future disc shaped?Biochem Soc Trans. 2016 Aug 15;44(4):1011-8. doi: 10.1042/BST20160015. Biochem Soc Trans. 2016. PMID: 27528746 Review.
-
GPCR-styrene maleic acid lipid particles (GPCR-SMALPs): their nature and potential.Biochem Soc Trans. 2016 Apr 15;44(2):619-23. doi: 10.1042/BST20150284. Biochem Soc Trans. 2016. PMID: 27068979 Review.
Cited by
-
Lipid Dynamics in Diisobutylene-Maleic Acid (DIBMA) Lipid Particles in Presence of Sensory Rhodopsin II.Int J Mol Sci. 2021 Mar 4;22(5):2548. doi: 10.3390/ijms22052548. Int J Mol Sci. 2021. PMID: 33806280 Free PMC article.
-
Use of paramagnetic systems to speed-up NMR data acquisition and for structural and dynamic studies.Solid State Nucl Magn Reson. 2019 Oct;102:36-46. doi: 10.1016/j.ssnmr.2019.07.002. Epub 2019 Jul 12. Solid State Nucl Magn Reson. 2019. PMID: 31325686 Free PMC article. Review.
-
Kinetic analysis of antibody binding to integral membrane proteins stabilized in SMALPs.BBA Adv. 2021 Aug 5;1:100022. doi: 10.1016/j.bbadva.2021.100022. eCollection 2021. BBA Adv. 2021. PMID: 37082021 Free PMC article.
-
Fabrication of Eco-Friendly Hydrolyzed Ethylene-Maleic Anhydride Copolymer-Avermectin Nanoemulsion with High Stability, Adhesion Property, pH, and Temperature-Responsive Releasing Behaviors.Molecules. 2024 Mar 5;29(5):1148. doi: 10.3390/molecules29051148. Molecules. 2024. PMID: 38474660 Free PMC article.
-
Current Developments in Native Nanometric Discoidal Membrane Bilayer Formed by Amphipathic Polymers.Nanomaterials (Basel). 2021 Jul 7;11(7):1771. doi: 10.3390/nano11071771. Nanomaterials (Basel). 2021. PMID: 34361157 Free PMC article. Review.
References
-
- Baruah SD, Laskar NC, 1996. Styrene-maleic anhydride copolymers: synthesis, characterization, and thermal properties. J. Appl. Polym. Sci 60 (5), 649–656.
-
- Budil DE, Lee S, Saxena S, Freed JH, 1996. Nonlinear-Least-Squares Analysis of Slow-Motion EPR Spectra in One and Two Dimensions Using a Modified Levenberg-Marquardt Algorithm. J. Magn. Reson. A 120, 155–189.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

