Cocaine abuse and midbrain circuits: Functional anatomy of hypocretin/orexin transmission and therapeutic prospect
- PMID: 30796894
- PMCID: PMC6702109
- DOI: 10.1016/j.brainres.2019.02.026
Cocaine abuse and midbrain circuits: Functional anatomy of hypocretin/orexin transmission and therapeutic prospect
Abstract
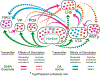
Cocaine abuse remains a pervasive public health problem, and treatments thus far have proven ineffective for long-term abstinence maintenance. Intensive research on the neurobiology underlying drug abuse has led to the consideration of many candidate transmitter systems to target for intervention. Among these, the hypocretin/orexin (hcrt/ox) neuropeptide system holds largely untapped yet clinically viable therapeutic potential. Hcrt/ox originates from the hypothalamus and projects widely across the mammalian central nervous system to produce neuroexcitatory actions via two excitatory G-protein coupled receptor subtypes. Functionally, hcrt/ox promotes arousal/wakefulness and facilitates energy homeostasis. In the early 2000s, hcrt/ox transmission was shown to underlie mating behavior in male rats suggesting a novel role in reward-seeking. Soon thereafter, hcrt/ox neurons were shown to respond to drug-associated stimuli, and hcrt/ox transmission was found to facilitate motivated responding for intravenous cocaine. Notably, blocking hcrt/ox transmission using systemic or site-directed pharmacological antagonists markedly reduced motivated drug-taking as well as drug-seeking in tests of relapse. This review will unfold the current state of knowledge implicating hcrt/ox receptor transmission in the context of cocaine abuse and provide detailed background on animal models and underlying midbrain circuits. Specifically, attention will be paid to the mesoaccumbens, tegmental, habenular, pallidal and preoptic circuits. The review will conclude with discussion of recent preclinical studies assessing utility of suvorexant - the first and only FDA-approved hcrt/ox receptor antagonist - against cocaine-associated behaviors.
Keywords: Addiction; Cocaine; Dopamine; Hypocretin/orexin; Midbrain; Psychostimulant(s); Reward; Ventral tegmental area.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures


References
-
- Ahmed SH, & Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science, 282(5387), 298–300. - PubMed
-
- Avvisati R, Contu L, Stendardo E, Michetti C, Montanari C, Scattoni ML, & Badiani A (2016). Ultrasonic vocalization in rats self-administering heroin and cocaine in different settings: evidence of substance-specific interactions between drug and setting. Psychopharmacology, 233(8), 1501–1511. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

